Macromolecules summary chart student driven answers
advertisement

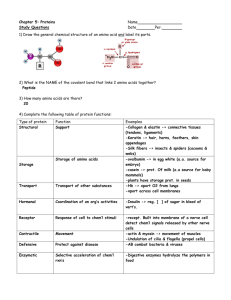
Macromolecules Summary Chart Macromolecule Subunits Functional groups Properties? (such as polar, nonpolar, solubility etc…) Hydroxyl -Polar (and carbonyl in linear form) -water soluble Monosaccari -des (simple sugars) Hydroxyl -Polar Monosaccari -des (simple sugars) Hydroxyl Carbohydrates 1.Monosaccarides 2. Oligosaccharides 3. Polysaccarides (McGraw-Hill Textbook p. 18-29) Type of linkage/ bond Type of reaction that makes polymer Examples Role/function Glucose , fructose, galactose Energy source Glycosidic Condensation (anabolic) Lactose, sucrose, maltose Ex: Sucrose transports glucose in maple trees Glycosidic Condensation (anabolic) 1. Starch (amylose = unbranched and amylopectin = branched) 1. energy storage for plants 2. Glycogen 2. energy storage for animals 3. Cellulose 3. Structural support for plants (dietary fibre) -water soluble (but not as much as a mono.) -Polar properties -loss of solubility due to length 4. Chitin 4. Exoskeletons for lobsters, crabs, bugs Macromolecules Summary Chart (McGraw-Hill Textbook p. 18-29) Macromolecule Subunits (General Structure) Functional groups Properties? (such as polar, nonpolar, solubility etc…) Type of linkage/bond Type of reaction that makes polymer Examples Role/function Lipids 1. Fats Glycerol + 3 fatty acids Glycerol has hydroxyl, fatty acids have carboxyl -non-polar -NOT soluble in water BUT soluble in oil ester Condensatio n (anabolic) 1. Saturated fats 1. all single bonded carbons. Solid at room temperature. Butter, lard 2. Phospholipids Glycerol + 2 fatty acids + phosphate head 3. Waxes Long chain fatty acids Glycerol has hydroxyl, fatty acids have carboxyl and head is made of phosphates -tail (fatty acids) is non-polar and thus water insoluble -Head is polar and water soluble Non-polar so not soluble in water 2. Unsaturated fats ester Condensatio n (anabolic) Can also be saturated or unsaturated Cutin on fruits Wax on feathers Honeycombs 2. Contain double and/or triple bonds, likely liquid at room temperature. Olive oil, sunflower oil etc… ALL FATS: Energy storage and insulation Make up the cellular membrane. Holds the cell together, lets things in a keeps things out Barrier to water Macromolecules Summary Chart 4. Steroids Can have hydroxyl and carbonyl Non-polar, not soluble in water (McGraw-Hill Textbook p. 18-29) Estrogen, testosterone, cholesterol Hormones, membrane structure Macromolecule Subunits Functional groups Properties? (such as polar, nonpolar, solubility etc…) Type of linkage/bond Type of reaction that makes polymer Examples Role/function Proteins 1. Linear polypeptide (fibrous) Amino acids All have carboxyl and amino. Some have hydroxyl or sulfhydryl All amino acids have polar properties some are more polar than others depending on the polarity of the R-group Peptide (aka amide) Condensatio n (anabolic) linear: Isulin, spider silk, keratin -Structural and functional Primary Secondary Macromolecules Summary Chart 2. Globular Tertiary Quaternary Amino Acid Amino aicds All have carboxyl and amino. Some have hydroxyl or sulfhydryl All amino acids have polar properties some are more polar than others depending on the polarity of the R-group (McGraw-Hill Textbook p. 18-29) Peptide (aka amide) Condensatio n (anabolic) globular: Collagen, haemoglobin, enzymes -Structural and functional Macromolecules Summary Chart Macromolecule Subunits Functional groups Nucleic Acids 1. DNA Nucleotides (phosphate + sugar + nitrogenous base) -Phoshpate 2. RNA Nucleotides (phosphate + sugar + nitrogenous base) Properties ? (such as polar, nonpolar, solubility etc…) (McGraw-Hill Textbook p. 18-29) Type of linkage/bond Type of reaction that makes polymer Role/function phosphodiester Condensatio n (anabolic) Informational (hereditary) phosphodiester Condensatio n (anabolic) Transmits hereditary information from nucleus to cytoplasm -Some nitrogenous bases have carbonyl and amino -sugars have hydroxyl Phoshpate -Some nitrogenous bases have carbonyl and amino -sugars have hydroxyl examples Macromolecules Summary Chart Nucleotide (McGraw-Hill Textbook p. 18-29)