Prototype DRUG: Cyclobenzaprine (Cycloflex, Flexeril)
advertisement

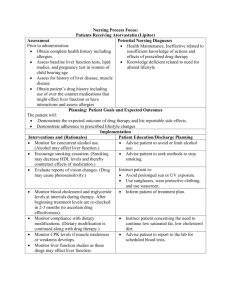
NURSING Process FOCUS: Patients Receiving Cyclobenzaprine (Cycloflex, Flexeril) Assessment Potential Nursing Diagnoses Prior to administration: Pain related to disease process Assess for pain, muscle stiffness, and Impaired Physical Mobility related to mobility (initially and throughout therapy) pain Obtain complete medical history, Knowledge Deficit related to action and especially renal, liver, neurological effects of medication diseases including blood studies: CBC, WBC with differential for blood dyscrasias and liver function studies Obtain patient’s drug history to determine possible drug interactions and allergies Planning: Patient Goals and Expected Outcomes The patient will: State relief of pain and spasms Report an increase in range of motion of affected body part State expected outcomes of drug therapy and list reportable side effects Implementation Interventions and (Rationales) Patient Education/Discharge Planning Instruct the patient to: Observe for side effects such as drowsiness, dry mouth, dizziness, nausea, Not drive or operate dangerous machinery vomiting, faintness, headache, when taking muscle relaxants. nervousness, diplopia, and urinary Take medication with food to decrease retention. gastrointestinal upset. Report signs of urinary retention such as feeling of fullness, distended abdomen, and discomfort. Use frequent mouth rinses and sugarless candy or gum if expereincing dry mouth Monitor serum liver enzymes levels. Instruct patient concerning the importance Report elevation (Medication may cause of having lab work done serious liver damage) Monitor length of time on drug. (Drug Inform the patient that the muscle should be tapered to avoid rebound relaxants should not be taken for more spasms.) than 3 weeks and should not be abruptly stopped. Evaluation of Outcome Criteria Evaluate the effectiveness of drug therapy by confirming that patient goals and expected outcomes have been met (see “Planning”). NURSING Process FOCUS: Patients Receiving Dantrolene Sodium (Dantrium) Assessment Potential Nursing Diagnoses Prior to administration: Pain related to disease process Assess for pain, muscle strength, and Impaired Physical Mobility related to spasticity (initially and throughout pain therapy) Self Care Deficit related to Immobility Obtain complete medical history, and pain especially cardiac, renal, liver, Knowledge Deficit related to action neurological diseases including blood and effects of medication studies: CBC, WBC with differential for blood dyscrasias and liver function studies Obtain patient’s drug history to determine possible drug interactions and allergies Assess ability to self administer medication Planning: Patient Goals and Expected Outcomes The patient will: State relief of pain and spasticity Report an increase in range of motion and dexterity of affected body part State expected outcomes of drug therapy and list reportable side effects Implementation Interventions and (Rationales) Patient Education/Discharge Planning Instruct the patient to: Observe for side effects such as muscle weakness, drowsiness, dry mouth, dizziness, Report side effects. nausea, diarrhea, tachycardia, erratic blood Not drive or operate dangerous pressure, photosensitivity, and urinary machinery when taking muscle retention. relaxants. Take medication with food to decrease gastrointestinal upset. Report signs of urinary retention such as feeling of fullness, distended abdomen, and discomfort. Use frequent mouth rinses, sips of water, and sugarless candy or gum may help with dry mouth Use sunscreen Monitor for muscle rigidity, pain, range of Inform patient to report changes in motion, and dexterity (to monitor spasticity, range of motion, dexterity, effectiveness of medication). and pain levels to order to determine effectiveness. Monitor serum liver enzymes levels. Report Instruct patient concerning the elevation. importance of having lab work done because medication may cause serious liver damage. Monitor length of time on drug. (Drug Inform the patient that the muscle should be tapered over a one to two week relaxants should not be abruptly period to avoid rebound spasms.) stopped. Monitor ability to take medication, assist if Instruct family or caregiver about necessary. (Spasms may inhibit ability of medication and proper administration in patient to self-administer medication) case patient is unable to self-medicate. Evaluation of Outcome Criteria Evaluate the effectiveness of drug therapy by confirming that patient goals and expected outcomes have been met (see “Planning”). NURSING PROCESS FOCUS PATIENTS RECEIVING CALCIUM SUPPLEMENTS ASSESSMENT POTENTIAL NURSING DIAGNOSES ■ Prior to administration: ■ Obtain complete health history including allergies, drug history, and possible drug Risk for Injury, related to loss of bone mass and side effects of drug ■ Deficient Knowledge, related to drug therapy interactions. ■ ■ Obtain baseline ECG. Obtain baseline vital signs, especially apical pulse for rate and rhythm, and blood pressure. ■ Obtain lab work to include CBC and electrolytes, especially calcium. PLANNING: PATIENT GOALS AND EXPECTED OUTCOMES The patient will: ■ ■ Have normal serum calcium levels (8.5–11.5 mg/dl) Demonstrate an understanding of the drug’s action by accurately describing drug side effects and precautions, and measures to take to decrease any side effects ■ Immediately report side effects and adverse reactions IMPLEMENTATION Interventions and (Rationales) Patient Education/Discharge Planning ■ ■ Monitor electrolytes throughout therapy. Teach patient of importance of routine lab studies, (Calcium and phosphorus levels tend to vary so deviations from normal can be corrected inversely. Low magnesium levels tend to immediately. coexist with low calcium levels.) ■ Monitor for signs and symptoms of ■ Instruct patient to report signs or symptoms of hypercalcemia. (Overtreatment may lead to hypercalcemia: drowsiness, lethargy, weakness, excessive serum calcium levels.) headache, anorexia, nausea and vomiting, increased urination, and thirst. ■ ■ Initiate seizure precautions for patients at risk ■ Teach patient to be aware of signs of for hypocalcemia. (Low calcium levels may hypocalcemia, such as seizures, muscle spasms, cause seizures.) facial twitching, and paresthesias. Monitor for musculoskeletal difficulties. (Calcium gluconate is used to treat osteoporosis, Instruct patient to: ■ ■ rickets, osteomalacia.) Take special precautions to prevent fractures Report episodes of sudden pain, joints out of alignment, inability of patient to assume normal positioning ■ Monitor intake and output. Use cautiously in ■ patient with renal insufficiency. (Calcium is Instruct patient to report any difficulty in urination and measure I&O. excreted by the kidneys.) ■ Monitor cardiac functioning. (Possible side ■ effects may include short QT wave, heart block, Inform patient to recognize and report palpitations or shortness of breath to healthcare provider. hypotension, dysrhythmia, or cardiac arrest with IV administration.) ■ Monitor injection site during intravenous ■ Instruct patient to report any pain at IV site. administration for infiltration. (Extravasation may lead to necrosis.) ■ Monitor diet. (Consuming calcium-rich foods may increase effect of drug. Consuming foods ■ Advise patient to: consume calcium-rich foods and avoid zinc-rich foods rich in zinc may decrease calcium absorption.) EVALUATION OF OUTCOME CRITERIA Evaluate the effectiveness of drug therapy by confirming that patient goals and expected outcomes have been met (see “Planning”). Nursing Process Focus: Patients Receiving CALCITRIOL (Rocaltrol, Calcijex) Assessment Potential Nursing Diagnoses Prior to administration: Comfort, impaired, related to effects of calcitriol Knowledge, deficient, related to no previous contact with calcitriol Therapeutic regimen management, ineffective related to length of time treatment is necessary Urinary elimination, modified, urinary frequency, related to effects of medication Obtain complete health history including allergies, drug history and possible drug interactions Assess for presence/history of hypercalcemia, hypocalcemia, vitamin D toxicity, parathyroid dysfunction, decreased renal function. Assess lab values of electrolytes, cholesterol, alkaline phosphatase, calcium and creatinine Planning: Patient Goals and Expected Outcomes Patient will: Demonstrate increased bone mass Demonstrate knowledge of dietary modifications to include adequate calcium and Vitamin D Demonstrate understanding of drug treatment regimen Remain free of physical discomfort Maintain urinary elimination within normal limits Implementation Interventions and (Rationales) Patient Education/Discharge Planning *Evaluate patient knowledge about proper storage. (Improper storage will render calcitriol ineffective.) *Instruct patient to protect medication from light, heat and moisture. *Monitor diet for adequate calcium and phosphate content. (Effectiveness of calcitriol therapy depends on adequate daily intake of calcium and phosphate.) Advise patient: *Monitor vitamin D intake. (Excessive intake of vitamin D may lead to hypercalcemia.) to include foods high in calcium and phosphate to avoid foods high in sodium or potassium to increase fluids, unless advised not to by health care provider symptoms of hypercalcemia, deep bone and flank pain, anorexia, nausea and vomiting, unusual thirst, constipation, lethargy, psychosis *Advise patient to avoid any other sources of vitamin D therapy while taking calcitriol. *Monitor for side effects/adverse reactions. *Instruct patient and caregiver to monitor for and report: headache, weakness, irritability, somnolence, conjunctivitis, photophobia, rhinorrhea, anorexia, nausea/vomiting, constipation, weight loss, polydipsia, dry mouth, metallic taste, polyuria, nocturia, bone and muscle pain, pruritis, decreased libido. Evaluation of Outcome Criteria Evaluate the effectiveness of drug therapy by confirming that patient goals and expected outcomes have been met (see “Planning”). Nursing Process Focus: Patients Receiving RALOXIFENE (Evista) Assessment Potential Nursing Diagnoses Prior to administration: Comfort, impaired, related to side effects of raloxifene Obtain complete health history including allergies, drug therapy and Fluid volume, excess, related to possible drug interactions water and sodium retention secondary to medication Assess for presence, history of pregnancy, venous thrombosis, Knowledge, deficient, related to no pulmonary emboli, hnormone use, previous contact with breast abnormalities medication Obtain vital signs Nausea, related to side effects of raloxifene Obtain history of cardiovascular or cerebrovascular disease Thought processes, disturbed, depression, related to side effects of medication Planning: Patient Goals and Expected Outcomes Patient will: Demonstrate bone density within normal limits Demonstrate understanding of need for long-term compliance with medication regime Demonstrate understanding of side effects/adverse reactions to report to health care provider Maintain intact thought processes and absence of symptoms of depression Implementation Interventions and (Rationales) *Monitor bone density tests and liver function tests. Patient Education/Discharge Planning *Instruct patient to have bone density tests and liver function tests prior to beginning raloxifene and periodically during therapy. *Monitor for thromboembolism. (This may *Advise patient about symptoms to report indicate need to discontinue therapy.) immediately: pain in calf; sudden dyspnea accompanied by feeling of breathlessness and impending doom; vision changes. *Monitor weight. (Edema may appear, and Teach patient and caregivers: cause weight gain.) how to perform accurate weights to report weekly weight gain >5 lbs *Monitor activity level. (Prolonged periods Advise patient: of immobility may increase risk of to avoid sitting in one position for long thromboembolism.) period to increase exercise if able and to do weight-bearing exercises, or to use weights when exercising *Obtain smoking history. (Smoking has an Encourage patient: inverse effect on bone density.) to stop smoking to attend smoking-cessation clinics, courses, etc. *Monitor for hot flashes. (Raloxifene does Teach patient: not prevent, and may induce, hot flashes.) that raloxifene does not help decrease severity of hot flashes measures that may increase comfort: dressing in layers, explaining physiology of hot flashes, drinking cool liquids, keeping thermostats turned lower than normal, etc. *Monitor for GU complaints. Patient may *Advise patient to increase fluid intake, experience breast pain, vaginal burning, or drink cranberry juice, practice careful itching, UTI. (Raloxifene exhibits selective personal hygiene, wear a supportive bra. estrogen receptor antagonist activity on breast and uterus.) *Monitor diet for calcium content. (It may *Advise patient to consume foods with be recommended that patient take a high calcium content, especially milk and calcium supplement if diet is low in milk products, and leafy green vegetables. calcium.) Evaluation of Outcome Criteria Evalaute the effectiveness of drug therapy by confirming that patient goals and expected outcomes have been met (see “Planning”). NURSING PROCESS FOCUS: Patients Receiving Etidronate (Didronel) Assessment Potential Nursing Diagnoses Prior to administration: Knowledge, deficient, related to no prior exposure Obtain complete health history including allergies, drug history and Fluid volume, imbalanced, risk for, possible drug interactions related to adverse reaction to medication Assess for presence/history of pathologic fractures, hypocalcemia, Nausea, related to side effects of hypercalcemia medication Assess nutritional status Pain, acute, bone, related to adverse reaction to etidronate Obtain lab work to include complete blood count, pH, electrolytes and renal Therapeutic regimen management, function studies (BUN, creatinine, uric ineffective, related to fact that acid) therapeutic response may take 1-3 months Assess lab values of calcium and phosphorous Planning: Patient Goals and Expected Outcomes Patient will: Demonstrate decreased progression of osteoporosis or Paget’s disease Demonstrate decreased risk for pathologic fractures Remain free of side effects or adverse reactions Demonstrate understanding of dietary needs/modifications Maintain adequate fluid volume Implementation Interventions and (Rationales) *Monitor for pathologic fractures and bone pain. (Etidronate causes defective mineralization of newly formed bone.) Patient Education/Discharge Planning *Instruct patient and caregiver to report any sudden bone or joint pain, inability of patient to correctly position self, swelling over bone or joint. *Monitor for GI problems. (There may be *Advise patient and caregiver that new problems with etidronate absorption if onset nausea or diarrhea may be symptom patient has persistent nausea or diarrhea.) of adverse reaction, and to report immediately. *Monitor serum calcium lab values: Advise patient: Serum calcium levels should be 9-10mg/dl. to have lab studies performed prior to (Through its inhibition of bone resorption, beginning etidronate therapy and etidronate causes blood levels of calcium periodically during therapy to fall.) symptoms of hypocalcemia to report (muscle spasms, facial grimacing, convulsions, irritability, depression, psychoses) symptoms of hypercalcemia to report (increased bone pain, anorexia, nausea/vomiting, constipation, thirst, lethargy, fatigue, confusion, depression) *Monitor kidney function, especially creatinine. (Etidronate cannot be used in patients whose creatinine is >5.) *Monitor BUN, vitamin D, urinalysis, phosphate, magnesium levels. *Monitor for side effects/adverse reactions including new onset nausea or diarrhea, metallic taste, constipation, stomatitis, fluid overload, chest pain, dyspnea, seizures. *Monitor dietary habits. (Diet must have adequate amounts of vitamin D, calcium and phosphates.) *Instruct patient and caregiver to report any urinary changes, such as decreased urine production, increased urination. *Instruct patient what symptoms to be aware of, and to report onset of them immediately. *Advise patient to include good food sources of vitamin D, calcium and phosphates, including dairy products and green leafy vegetables. *Monitor compliance with recommended Advise patient: regime. o that therapy should continue for 6 months maximum, but full therapeutic response may take 1-3 months o that effects continue several months after drug is discontinued o to avoid vitamins, mineral supplements, antacids and high-calcium products within 2 hours of taking etidronate. (All bisphosphonates are poorly absorbed from the GI tract. Measures should be taken to maximize absorption.) Evaluation of Outcome Criteria Evaluate the effectiveness of drug therapy by confirming that patient goals and expected outcome have been met (see “Planning”). Nursing Process Focus: Patients Receiving HYDROXYCHLOROQUINE SULFATE (Plaquinil Sulfate) Assessment Potential Nursing Diagnoses Prior to administration: Fluid volume, deficient, related to diarrhea, anorexia, nausea, vomiting Obtain complete health history including allergies, drug history and secondary to medication possible drug interactions Knowledge, deficient, related to no previous contact with this Assess for presence/history of malaria, rheumatoid arthritis, lupus medication erythematosus Nutrition, imbalanced, less than Assess mental status body requirements, related to anorexia, nausea, vomiting, diarrhea Assess GI status secondary to medication Assess CBC, platelets, liver function Sensory perception, disturbed, tests, vision and hearing tests G6PD vision and/or hearing, related to deficiency, muscle strength, reflexes, adverse reactions of medications EKG (depressed T waves, widening of QRS complex) Planning: Patient Goals and Expected Outcomes Patient will: experience no symptoms of malarial infection, decreased symptoms of rheumatoid arthritis with increased joint mobility, decreased symptoms of lupus erythematosus Demonstrate understanding of necessity of taking hydroxychloroquine exactly as ordered Recognize side effects and need to report Demonstrate understanding of necessity of follow-up appointments Implementation Interventions and (Rationales) *Monitor for hepatic problems, actual or potential. (Administer medication with caution to patients with decreased liver function, any patient taking hepatotoxic drugs, or alcoholic patient.) *Monitor patient and family knowledge regarding expected effects of medication. *Monitor for symptoms of toxicity. Patient Education/Discharge Planning *Instruct patient to report symptoms of liver dysfunction to health care provider. Advise patient and caregiver: that urine may turn rust or brown to wear sunglasses outside to decrease photo-phobia to report symptoms of blood dyscrasias (fever, fatigue, bruising, unusual bleeding *Instruct patient to discontinue drug and report to health care provider immediately if any of following occur: blurred vision and difficulty focusing, headache, dizziness, urticaria. Evaluation of Outcome Criteria Evalautge the effectiveness of drug therapy by confirming that patient goals and expected outcomes have been met (see “Planning”). NURSING PROCESS FOCUS: Patients Receiving Colchicine ASSESSMENT POTENTIAL NURSING DIAGNOSES Prior to administration: ■ ■ Obtain complete health history including allergies, ■ drug history, and possible drug interactions. ■ ■ ■ Obtain baseline vital signs. Activity Intolerance, related to joint pain Disturbed Body Image, related to joint swelling Deficient Knowledge, related to effects and side effects of drug therapy Obtain lab work to include CBC, platelets, uric acid levels, renal and liver function tests, and urinalysis. PLANNING: PATIENT GOALS AND EXPECTED OUTCOMES The patient will: ■ ■ Report a decrease in pain and an increase in function in affected joints Demonstrate an understanding of the drug’s action by accurately describing drug side effects and precautions, and measures to take to decrease any side effects ■ Immediately report side effects and adverse reactions IMPLEMENTATION Interventions and (Rationales) Patient Education/Discharge Planning ■ ■ Monitor lab results throughout therapy. Teach patient importance of routine lab studies, (Agranulocytosis and thrombocytopenia may so deviations from normal can be corrected occur.) Perform Coombs test for hemolytic immediately. anemia. ■ Monitor for signs of toxicity. ■ Instruct patient to report weakness, abdominal pain, nausea, and/or diarrhea. ■ Monitor for signs of renal impairment such as oliguria. Record intake and output. ■ Instruct patient to report a decrease in urinary output and to increase fluid intake to 3–4 L/day. ■ Ensure that medication is administered correctly. ■ Inform patient to take medication on an empty stomach. Medication should be taken at first sign of gout attack. ■ Monitor for pain and mobility. (This is used to assess effectiveness of medication.) ■ Teach patient to report an increase or decrease in discomfort and swelling. EVALUATION OF OUTCOME CRITERIA Evaluate the effectiveness of drug therapy by confirming that patient goals and expected outcomes have been met (see “Planning”).