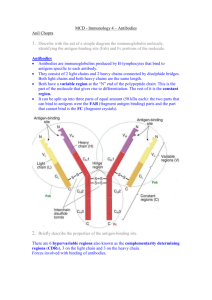
Antibody Structure: Antibodies consist of four polypeptides (two
advertisement

Chad Mosby Joe McClellan Cory Moore Luke Macon Antibody Final Model Project The immune system of the human body takes many effective defensive measures against foreign bodies. Antibodies play a pivotal role in the identification and elimination of such pathogens. We chose to create - upon Dr. Falvo’s suggestion - a model of an antibody because we wanted to learn more about its structure and how it relates to its function. Antibody Structure: Antibodies consist of four polypeptides (two heavy chains and two light chains), which are joined together to form a symmetrical y-shaped structure. The light chains (seen on the outsides of this diagram) are composed of 2 domains of approximately 110 amino acids each. The heavy chains in the center are composed of 4 or 5 domains of approximately 110 amino acids each. Each of these domains consists of 2 beta pleated sheets joined by a single disulfide bond. Both the light and the heavy chains have a variable domain on the end (shaded in blue) with three small hypervariable regions that determine the shape of the antigen-binding site. http://www.accessexcellence.org/RC/VL/GG/images/Fig_5. These hypervariable regions are 25.jpg short polypeptide sections of 5-10 amino acids that directly contact the antigen at the antigenic determinant (what the antibody recognizes and binds to). The rest of the long and short chains are constant regions from antibody to antibody. Antibodies have the ability to hinge at the disulfide bonds between Illustration of domains with β sheets from th the heavy and light chains in order to Molecular Biology of the Cell (4 edition) accommodate the size of the antigenic determinant. They also are able to hinge at the two disulfide bonds that connect the 2 heavy chains. How antibodies are produced: Antibodies are made by B lymphocytes. B cells begin in the bone marrow as preB cells. Eventually, after rearrangement of genes coding for immunoglobulin, antibodies are embedded in the cell membrane. The cell is now called a naïve B cell, because it has not yet interacted with antigen. All of the antibodies on one cell are identical, and all B Chad Mosby Joe McClellan Cory Moore Luke Macon cells in the body produce different antibodies by random genetic combination. At least 90% of B cells never interact with antigen and die within several days. When a B cell does come into contact with antigen, the antigen is brought inside the cell and processed for presentation to a T cell. When the B cell comes into contact with a T cell specific for the same antigen, the T cell releases cytokines, which cause the B cell to differentiate. The T cell cannot detect antigen in its pure form. It must have the antigen presented to it by an antigen presenting cell such as a macrophage that has ingested a bacteria. When the antigen is processed by the macrophage, it is broken up and displayed on the outside by Class II MHC (major histocompatability complex) proteins. Much like surface antibodies of a naïve B cell, the T cell has antigen receptors on the surface that are formed through random genetic combination. T cell activation occurs when these receptors interact with the antigen-MHC Class II molecules. Now the T cell is ready to interact with B cell. Activated B cells form plasma cells and memory cells. These new cells may differ in the class of antibody that they produce, but all will be specific to the same antigen. Plasma cells secrete large numbers of free antibodies, which circulate through the body. Some of the B cells maintain the IMGT Marie-Paule page presence of http://imgt.cines.fr/textes/IMGTeducation/T surface antibodies, utorials/IGandBcells/_UK/MolecularGenetic similar to the s/angfig5.html naïve B cells, and are known as memory cells. The presence of these cells improves immune response the next time the antigen is detected because they require lower levels of antigen to become activated. How antibodies function: The antibody eliminates pathogens in the body. It does this by binding to sites on the pathogen known as antigens. The hypervariable regions of the antibody bond with the antigenic determinant in an interaction that is made more specific by hydrogen bonds, van der Waals forces, hydrophobic interactions and electrostatic forces. Once an antibody has attached itself to the antigen, it can eliminate the foreign body in four different ways. First, if the foreign body is a virus, the antibody begins the process of neutralization, during which it blocks viral receptors from binding to their preferred Chad Mosby Joe McClellan Cory Moore Luke Macon docking site on a cell. This keeps the virus from injecting its genetic code into cells. The antibody can also cause agglutination of the foreign bodies, in which the intruders are clumped together and eaten by phagocytes. Antibodies can also just mark individual cells for ingestion by phagocytes. Alternatively, the antibody can activate a mechanism for destruction known as the membrane attack complex. Nanotechnology application: One potential use of antibodies in nanotechnology is improving methods of drug delivery. Antibodies known to target melanoma tumor cells can be combined with multiple C60 buckyballs in order to deliver multiple drugs simultaneously to the tumor without having to spread the medication throughout the entirety of the human body. Multiple buckyballs are used so that a variety of medicines can be delivered, increasing the chances that the cancer cells are totally eliminated. This specific delivery cuts down on the harmful side-effects of the medicine used to kill these tumor cells. In modeling an antibody, we used pipe cleaners to represent the polypeptide chains. The structure of the antibody includes many distinct shapes and the model therefore called for a material that was easy to manipulate. The malleability of the pipe cleaners allowed the construction and interconnection of the beta sheets that compose the antibody. This also assisted in making the hypervariable regions, disulfide bonds, and antigen portions of the model. Cardboard was used to create the antigens, differentiating the antigens from the antibody while still achieving a geometric complement to the hyper variable regions. Twist ties were then used inside the beta sheets in order to maintain stability. As we researched and created this antibody model, we discovered that many systems with many complex components must interact for the immune system to function properly. This gave us a greater appreciation for the essential service our immune system provides.