Immunology 4 – Antibodies
advertisement

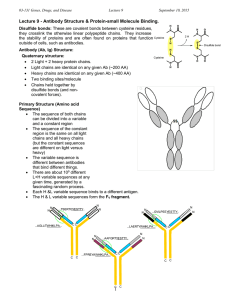
MCD - Immunology 4 – Antibodies Anil Chopra 1. Describe with the aid of a simple diagram the immunoglobulin molecule, identifying the antigen-binding site (Fab) and Fc portions of the molecule. Antibodies Antibodies are immunoglobulins produced by B-lymphocytes that bind to antigens specific to each antibody. They consist of 2 light chains and 2 heavy chains connected by disulphide bridges. Both light chains and both heavy chains are the same length. Both have a variable region at the “N” end of the polypeptide chain. This is the part of the molecule that gives rise to differentiation. The rest of it is the constant region. It can be split up into three parts of equal amount (50 kDa each): the two parts that can bind to antigens were the FAB (fragment antigen binding) parts and the part that cannot bind is the FC (fragment crystals). 2. Briefly describe the properties of the antigen-binding site. There are 6 hypervariable regions also known as the complementarity determining regions (CDRs). 3 on the light chain and 3 on the heavy chain. Forces involved with binding of antibodies. 3. Distinguish between antibody affinity and antibody avidity. Antibody Affinity: the strength of the total noncovalent interactions between a single antigen binding site and a single epitope on the antigen. Association constant K varies from 104 to 1011 L/mol. Antibody Avidity: the overall strength of multiple interactions between an antibody with multiple binding sites and a complex antigen with multiple epitopes. This is a better measure of binding capacity in biological systems. Antibody Cross-Reactivity: this where an antibody can recognise more than one antigen. E.g. antigen in response to cowpox also binds to smallpox antigen. Isotype: this refers to the structures of immunoglobulins which are present in everyone. (e.g. kappa and lambda light chains, the different heavy chains, the classes and sub-classes) Allotype: this refers to the different alleles of immunoglobulins that code for varying regions of their structure. These can be detected by other antibodies (e.g. -chain, Gm; -chain, km) 4. List the immunoglobulin classes and subclasses in man. Describe their functions and relate these to their individual structure. IgG and IgA also have sub classes: IgG: this is the most abubndant of all the immunoglobulins and is the most important in defence. It neutralises toxins, aids in phagocytosis, aids in the activation of complement. It also crosses the placenta into the foetal circulation giving the baby some immune protection in its first months of life. Has a - heavy chain. IgA: this contains an heavy chain and is the second most abundant immunoglobulin. It occurs as a monomer (blood) and as a dimer (secretions). It is the major secretory immunoglobulin and it protects mucosal surfaces from bacteria, viruses and protozoa. It binds to the infections agents blocking their infectivity, and also neutralises bacterial and viral toxins. IgM: this has a heavy chain. It is a large pentameric molecule (i.e. it has 5 monomers joined by J chain -10 x Fab). It is mainly found in the blood (80%), and it is the first Ig synthesised after exposure to antigen. It has a low antibody affinity, but a high avidity with its numerous binding sites, which also make it very effective at agglutination. It also activates compliment. IgD: this has heavy chain. It has extremely low serum concentrations and is the least well characterised immunoglobulin. Surface IgD expressed early in B cell development. IgE: this has a heavy chain. It is present at extremely low levels and is only produced in response to parasitic infections and in allergic diseases. It binds to high affinity Fc receptors of mast cells and basophils. Its cross-linking by various antigens triggers mast cell activation and histamine release.