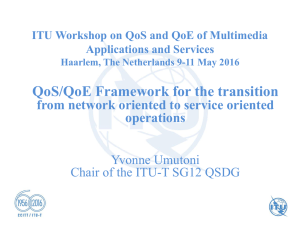
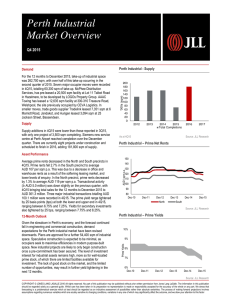
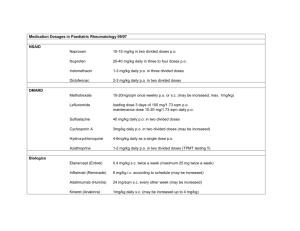
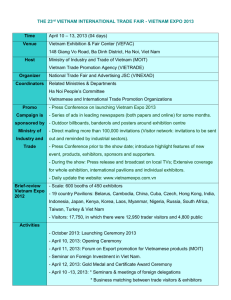
NPM1 mutations in familial acute myeloid leukemia

SUPPLEMENTARY MATERIALS
THERAPEUTIC REGIMENS IN THE FATHER
Induction and consolidation therapy (father)
Cytosine arabinoside (ARA-C) (100 mg/sqm continuous infusion; 200 mg total)
(April 6-May 6, 2006), VP16-Etoposide (100 mg/sqm i.v.; 200 mg total) (April 27-May 1 st ,
2006), and Daunomycin (DNM) (50 mg/sqm i.v.; 100 mg total) (April 27-29 and May 1 st ,
2006). Bone marrow examination after induction therapy showed complete haematological remission. Consolidation therapy consisted of ARA-C (500 mg/sqm i.v.; 950 mg total)
(June 6-9, 2006), DNM (50 mg/sqm i.v.; 95 mg total) (June 9-11, 2006), G-CSF s.c. (June
25-July 3, 2006). A bone marrow biopsy performed on July 12, 2006 confirmed complete haematological remission.
HLA-identical PBSC transplantation (father)
The father was transplanted from his HLA-identical brother at the time of first complete remission. Transplantation was performed as follows:
Conditioning regimen (Bu-Cy3)
Busulphan 4 mg/Kg/day orally (September 6-9, 2006)
(dose/die: 352 mg; total dose: 1408 mg)
Cyclophosphamide 50 mg/Kg/day i.v. (September 10-12, 2006)
(dose/die: 4400 mg; total dose 13200 mg)
Prophylaxis: Mesna 120% + LVC
Total body irradiation (TBI) was not administered
GVHD prophylaxis
Cyclosporin A: 3 mg/Kg/die i.v., starting at day -2
Methotrexate: 15 mg/sqm i.v., day + 2 (September 16); 10 mg/sqm e.v., day +4
(September 18); 10mg/sqm i.v., day +7 (September 21). The fourth dose at day + 12 was not administered because of fever and probable CVC infection.
Cells infused from the donor (the brother)
The followings cells were infused on September 14, 2006:
1
CNT (2.9 x 10 8 /Kg)
CD34+ (2.4 x 10 6 /Kg
CD3+ (5.9 x 10 6 /Kg)
+ CFU-GM
Complications
CVC infection (blood culture positive for Staphylococcus epidermis beta-lattamasi +)
Acute GVHD, grade III
THERAPEUTIC REGIMENS IN THE DAUGHTER
Front-line protocol (daughter)
Induction Phase: 2 courses of ICE:
Idarubicin 10 mg/sqm/daily intravenously for 3 days;
Cytosine Arabinoside 200 mg/sqm/daily i.v. for 7 days;
Etoposide 100 mg/sqm/daily i.v. for 5 days.
Consolidation Phase: 1 course of AVE:
Cytosine Arabinoside 3 gr/sqm/each 12 hours i.v. for 3 days;
Vepeside-Etoposide 125 mg/sqm/daily i.v. for 4 days.
1 course of HAM:
Cytosine Arabinoside 3 gr/sqm/each 12 hours i.v. for 3 days;
Mitoxantrone 10 mg/sqm/daily i.v. for 2 days.
Autologous PBSC transplantation (daughter)
Conditioning regimen
Busulphan 16 mg/Kg/day orally (days -8 to -5)
Cyclophosphamide 60mg/Kg/day i.v. (days -4 and -3)
LPAM 140 mg/sqm i.v. at day -2
At day O, infusion of mafosfamide-purged autologous MNC (2.9 x 10 8 /Kg)
2
First haploidentical stem cell transplantation (daughter)
Conditioning regimen
Total body irradiation (TBI): total of 12 Gy. Two fractions of 2 Gy/die (days -11 to -9)
Thiotepa: total dose of 10 mg/Kg (administered over days -8 to -7)
Fludarabine: total dose of 200 mg/sqm (administered over days -7 to -3)
Rabbit anti-lymphocyte globulin (ATG Fresenius): total dose of 25 mg/Kg
(administered over days -6 to -2).
At day 0 (May 7, 2007), infusion of a total of 8.41 x 10 6 /Kg cells from the mother
(consisting of: 8.29 x 10 6 /Kg CD34+ cells, 6.3 x 10 4 /Kg CD20+ cells, 0.83 x 10 4 /Kg
CD3+ cells). Engrafment was at day 14.
No post-transplantation prophylaxis of GvHD was given.
Complications
Fever at day -6 during infusion of ATG
At day +3 following infusion, CVC infection (blood culture positive for E. Coli )
Second haploidentical stem cell transplantation (daughter)
In March 2008, the child received unmanipulated bone marrow stem cells from the same donor (the mother) after a conditioning that included treosulfan, thiotepa and clofarabine.
She developed grade II acute GvHD and achieved a CR with a full-donor type chimerism.
3