Cell Free Reagent Preparation Protocol
advertisement

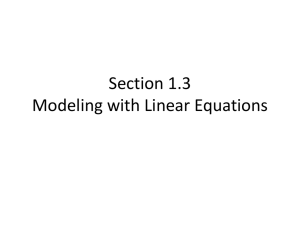
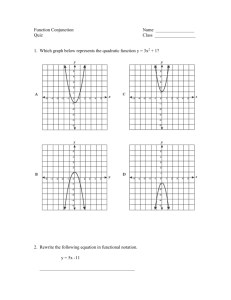
Supplementary Materials: PANOx-SP Premix Protocols Supplementary Materials Simplifying and streamlining E. coli-based cell-free protein synthesis Preparation of a PANOx-SP premix solution for cell-free protein synthesis 1. Introduction This section details the procedures for the preparation of individual cell-free protein synthesis (CFPS) precursor solutions and combination of these solutions into 500-mL of a convenient 4x premix (Figure S1). 500-mL of the 4x premix is sufficient to perform 2L of CFPS. If volumes smaller or larger volumes than 500-mL are desired, the amount of reagents and chemicals must be scaled down or up accordingly. The precursor solutions have typically been prepared using the following protocols in the following order: 1) Preparation of a Magnesium Glutamate Solution 2) Preparation of a 10x Salt Solution 3) Preparation of a 25x Nucleotide Mix 4) Preparation of a 25x 19 amino acid mix 5) Preparation of a 25x PEP solution 6) Testing of individual CFPS precursor solutions (Test batch of 4x Premix) 7) Combination of individual CFPS precursor solutions into a Final 4x Premix solution Equipment Note: Using a heated stir plate with temperature control along with a pH probe and pH meter will be helpful in preparing the CFPS precursor solutions and Premix. 1 Supplementary Materials: PANOx-SP Premix Protocols (2) Prepare Test 4x Premix (1) Prepare precursor solutions, aliquot, and freeze at -80° C MgGlu 50µL 10x SS MgGlu Magnesium Glutamate 25x N-Mix MgGlu rest 25x AA 250µL 10x Salt Solution (SS) Thaw small aliquots, mix, and make Test 4x Premix 25x PEP 10x SS Perform CFPS with Test 4x Premix and identify problematic reagents (if any) 10x SS rest 100µL 25x Nucleotide Mix (N-Mix) 25x N-Mix 25x N-Mix rest 100µL 25x 19 Amino Acid Mix (AA) (3) Prepare Final 4x Premix MgGlu 25x AA 10x SS 25x AA rest 25x N-Mix 100µL Thaw large aliquots and make Final 4x Premix 25x PEP Aliquot Final 4x Premix, freeze, and store at -80° C 25x AA 25x PEP 25x PEP 25x PEP rest Perform CFPS with Final 4x Premix to confirm protein synthesis activity Figure S1: Flow diagram for preparation of a 4x premix solution for CFPS. 2. Preparation of a Magnesium Glutamate Solution (The following protocol describes the preparation of 44 mL of 1000 mM magnesium glutamate solution) Magnesium glutamate is kept as a separate addition because the magnesium concentration for maximum protein production varies from one lot of cell extract to another. The typical optimal magnesium glutamate concentration for PANOx-SP is in between 10-20 mM. The default magnesium glutamate for the preparation described in this work is 10 mM. Therefore, additional magnesium glutamate may need to be added to reach the optimum concentration depending on the particular extract that is used. 2 Supplementary Materials: PANOx-SP Premix Protocols Reagent L-Glutamic Acid, Hemimagnesium Tetryhydrate (Magnesium Glutamate) Formula Weight (g/mol) 388.6 Main Precursor Concentration 1000 mM Concentration in CFPS Required Quantity (g) TBD 17.1 Actual Quantity (g) Table S1: 1000 mM Magnesium Glutamate Component. 1) Determine the desired final volume of the magnesium glutamate solution based on the mass (44 mL). Determine the required quantity of magnesium glutamate (17.1 g). 2) Obtain an appropriately sized mixing vessel and place it on an Isotemp stir plate (Fisher). 3) Place a stir bar in the mixing vessel and set the heating temperature to 37 °C. 4) Add autoclaved MQ water to the vessel (75% of the final volume). 5) Set the stir rate to a high mixing rate, but avoid splashing. 6) Weigh out the required quantity of magnesium glutamate in a weigh boat and pour the quantity into the mixing vessel. 7) Allow the magnesium glutamate to dissolve at 37 °C for 10 minutes with stirring. 8) Determine the volume of the solution using a pipette or graduated cylinder. 9) Add autoclaved MQ water to obtain the desired final volume. Allow the solution to stir at room temperature for 1 – 2 minutes until fully homogeneous. 10) Determine the pH of the solution and record the value. The pH should be approximately 6.4 - 6.5. 11) Aliquot 50 µL into an eppendorf tube for subsequent reagent testing. 12) Aliquot the remaining solution into 50-mL conical tubes, flash freeze using liquid nitrogen and store at -80 °C. 3 Supplementary Materials: PANOx-SP Premix Protocols 3. Preparation of the 10x Salt Solution (The following protocol describes the preparation of 220 mL of 10x Salt Solution) Reagent L-Glutamic Acid, Monopotassium Salt (Potassium Glutamate) L-Glutamic Acid, Monopotassium Salt (Ammonium Glutamate) Potassium Oxalate, Monohydrate Formula Weight (g/mol) 203.2 Main Precursor Concentration 1750 mM Concentration in CFPS Required Quantity (g) 175 mM 78.3 164.2 100 mM 10 mM 3.6 184.2 27 mM 2.7 mM 1.1 Actual Quantity (g) Table S2: 10x Salt Solution Components. 1) Determine the desired final volume of the 10x salt solution (220 mL). Determine the required quantity of potassium glutamate, ammonium glutamate, and potassium oxalate (Table S2 provides the required masses to prepare 220 mL of a 10x Salt Solution). 2) Obtain an appropriately sized mixing vessel and place it on the stir plate. Place a stir bar in the mixing vessel. 3) Add autoclaved MQ water (45% of the desired final volume). Set the stir rate to a high mixing rate, but avoid splashing. Set the stir plate heating to 37 °C. 4) Weigh out the required quantity of potassium glutamate and add into the beaker. Subsequent reagents can be added immediately. 5) Weigh out the required quantity of ammonium glutamate and add into the beaker. 6) Weigh out the required quantity of potassium oxalate and pour into the beaker. 7) Allow the salts to dissolve at 37 °C for 10 minutes on the stir plate. 8) Determine the volume of the solution using a pipette or graduated cylinder. 9) Add autoclaved MQ water to obtain the desired final volume. 10) Allow the salts to dissolve at room temperature. This may take 10 minutes. 4 Supplementary Materials: PANOx-SP Premix Protocols 11) Obtain a pH reading for the solution once all components are completely dissolved. The pH should be approximately 7.2. 12) Aliquot 250 µL for subsequent reagent testing. 13) Aliquot the remaining solution into multiple 50 mL falcon tubes, flash freeze with liquid nitrogen, and store at -80 °C. 4. Preparation of a 25x Nucleotide Mix (The following protocol describes the preparation of 88 mL of 25x Nucleotide Mix) The Nucleotide Mix contains polycations, cofactors, and the nucleotide triphosphates and monophosphates required for cell-free protein synthesis. The polycations putrescine and spermidine are difficult to weigh out as solids, so concentrated precursor solutions must be prepared prior to making the 25x Nucleotide Mix. The procedures to do this are detailed in sections 5 and 6. Appropriate volumes of the concentrated precursor solutions are then added along with the appropriate masses of the other chemical components to form the 25x Nucleotide Mix, detailed in section 7. Reagent Formula Weight (g/mol) Concentration in CFPS Required Volume (mL) of Precursor Solution 88.2 Desired Concentration in 25x Nucleotide Mix 25 mM 1000 mM 1,4Diaminobutane Solution (Putrescine) 1500 mM Spermidine Solution Reagent 1 mM 2.2 145.2 37.5 mM 1.5 mM 2.2 Formula Weight (g/mol) Concentration in CFPS Required Mass (g) 663.4 Desired Concentration in 25x Nucleotide Mix 8.3 mM Nicotinamide Adenine Dinucleotide (NAD) Adenosine 5’Triphosphate Disodium Salt (ATP) 0.33mM 0.5 551.1 30 mM 1.2 mM 1.5 5 Actual Volume (mL) of Precursor Solution Actual Mass (g) Supplementary Materials: PANOx-SP Premix Protocols Cytidine 5′monophosphate disodium salt (CMP) Guanosine 5′monophosphate disodium salt hydrate (GMP) Uridine 5′monophosphate disodium salt (UMP) Coenzyme A Hydrate (CoA) MRE600 E.coli tRNA (tRNA) Folinic Acid, Calcium Salt 367.16 21.5 mM 0.86 mM 1.0 407.18 21.5 mM 0.86 mM 1.0 368.15 21.5 mM 0.86 mM 1.0 767.5 6.8 mM 0.27 mM 0.5 N/A 4.3 mg/mL 170 µg/mL 0.4 511.5 0.9 mg/mL 34 µg/mL 0.1 Table S3: 25x Nucleotide Mix Components. 5. Preparation of a 1000 mM 1,4-diaminobutane (Putrescine) precursor solution (The following protocol describes the preparation of 284 mL of 1000 mM putrescine from a 25.0 g bottle of 1,4-diaminobutane, otherwise known as putrescine) 1) Determine the final volume for the 1000 mM 1,4-Diaminobutane precursor solution based on the mass (284 mL). 2) Obtain a 400 mL beaker and place it on ice. Place a stir bar in the beaker. 3) Add 20 mL autoclaved MQ water directly to the bottle of 1,4-diaminobutane. 4) Vortex the bottle vigorously for 1-2 minutes to facilitate dissolution. Pour out the liquid from the bottle into the beaker. 5) Repeat steps 3 and 4 two times. After adding the final 20 mL of Autoclaved MQ water, let the water in the bottle sit on ice for 10 – 15 minutes. 6) Pour out the liquid from the bottle into the beaker. At this point the 1,4-diaminobutane should be completely dissolved or the solid should have dislodged from the bottle. As needed, more water can be added to dissolve any remaining reagent. However, do not exceed the desired final volume. 7) Once the 1,4-Diaminobutane has dissolved, rinse out the bottle with 10 mL of autoclaved MQ water and pour it into the beaker. 8) Determine the volume of the solution using a pipette or graduated cylinder. 6 Supplementary Materials: PANOx-SP Premix Protocols 9) Add autoclaved MQ water to obtain the desired final volume. Place the beaker onto a stir plate. Allow the solution to stir at room temperature for 1 - 2 minutes. 10) The pH should be approximately 13. 11) Leave the solution on ice for immediate use or aliquot in multiple 50-mL conical tubes. If you choose to aliquot, then flash freeze using liquid nitrogen and store at -80 °C. 6. Preparation of a 1500 mM Spermidine precursor solution (The following protocol describes the preparation of 23 mL of 1500 mM spermidine from a 5.0 g bottle of spermidine) 1) Determine the final volume for the 1000 mM spermidine precursor solution based on the mass (23 mL). 2) Obtain a beaker and place it onto a stir plate with a stir bar. 3) Add 5 mL autoclaved MQ water to the bottle of spermidine. 4) Shake/vortex the bottle vigorously for 1 – 2 minutes to facilitate dissolution. Pour out the liquid from the bottle into the mixing vessel. 5) Repeat steps 3 and 4. 6) Once all of the spermidine has dissolved rinse out the bottle with autoclaved MQ water and pour it into the mixing vessel. 7) Determine the volume of the solution using a pipette or graduated cylinder. 8) Add autoclaved MQ water to obtain the desired final volume. Allow the solution to stir at room temperature for 1 – 2 minutes until fully homogeneous. 9) The pH of the solution should be approximately 13. 10) Leave the solution on ice for immediate use or aliquot in 50-mL conical tubes. If you choose to aliquot, then flash freeze using liquid nitrogen and store at -80 °C. 7. Preparation of the 25x Nucleotide Mix using the Putrescine and Spermidine precursor solutions 1) Determine the desired final volume for the 25x Nucleotide Mix solution (88 mL). Determine the required quantity of each of the liquid and solid reagents (Table S3 provides the required volumes and masses for preparing 88 mL of a 25x Nucleotide Mix). 7 Supplementary Materials: PANOx-SP Premix Protocols 2) Obtain an appropriately sized mixing vessel and place it onto a stir plate. Place a stir bar into the bottom of the mixing vessel. 3) Add 50% of the final volume, autoclaved MQ water to the vessel. 4) Set the stir rate to a sufficient mixing rate and such that splashing does not occur. 5) Add the required volume of the 1000 mM putrescine precursor solution to the mixing vessel. 6) Add the required volume of the 1500 mM spermidine precursor solution to the mixing vessel. 7) Weigh out the required quantity of NAD in a weigh boat and pour the quantity into the beaker. If lyophilized NAD in 50 mg vials are used, add 1mL of autoclaved MQ water to each vial that is needed. Vortex until fully dissolved. Add the appropriate volume of NAD solution. Assume that the contents of each vial, once dissolved, contains 50 mg of NAD. 8) Ensure the complete dissolution of the material before proceeding with the next step. 9) Add the required quantity of CoA into the beaker. 10) Ensure the complete dissolution of the material before proceeding with the next step. 11) Add the required quantity of to the beaker. 12) Ensure the complete dissolution of the material before proceeding with the next step. 13) Add the required quantity of ATP into the beaker. If whole vials of ATP are used, 1 mL of water may be used to rinse out each of the emptied vial, previously containing 1 g ATP. 14) Add CMP, GMP, and UMP in that order, in a fashion similar to the ATP described above. Ensure the complete dissolution of the material before proceeding with the next step. 15) Add the required quantity of Folinic Acid in a weigh boat and pour the quantity into the beaker. 16) Determine the volume of the solution using a pipette or graduated cylinder. Record the quantity. 17) Add autoclaved MQ water to obtain the desired final volume. 18) Determine the pH of the solution and record the value. The pH should be approximately 7.4 - 7.6. 8 Supplementary Materials: PANOx-SP Premix Protocols 19) Aliquot 100 µL for reagent testing. Aliquot the remaining solution into multiple 50-mL conical tubes, flash freeze with liquid nitrogen, and store at -80 °C. 8. Preparation of 25x 19 Amino Acid Mix (50mM of each amino acid) (The following describes the preparation of 200 mL of 25x 19 Amino Acid Mix) This is the precursor solution of amino acids that will be used by the CFPS reaction. Glutamate is omitted from this precursor because it will already be present in the form of potassium glutamate in the previously prepared 10x Salt Solution. Order of Addition / Reagent Formul a Weight (g/mol) Quantity (g) pH After Addition Dissoluti on Time (sec) Requir ed Approx. Approx. Actual Actual 1 L-Arginine 174.2 1.7420 10.65 – 10.68 30 2 L-Valine 117.1 1.1710 8.99 – 9.20 180 3 L-Tryptophan 204.2 2.0420 8.66 – 8.71 300 4 LPhenylalanine 165.2 1.6520 8.42 – 8.46 300 5 L-Isoleucine 131.2 1.3120 8.31 – 8.34 480 6 L-Leucine 131.2 1.3120 8.22 – 8.25 480 7 L-Cysteine 121.2 1.2120 7.80 – 7.84 120 8 L-Methionine 149.2 1.4920 7.73 – 7.75 240 9 DL-Alanine 89.09 0.8909 7.76 – 7.77 30 10 L-Asparagine 132.1 1.3210 7.64 – 7.71 60 11 L-Aspartic Acid 133.1 1.3310 5.35 – 5.41 180 12 L-Glycine 75.07 0.7507 5.34 – 5.42 60 13 L-Glutamine 146.1 1.4610 5.34 – 5.40 120 14 L-Histidine 155.2 1.5520 6.46 – 6.54 240 15 L-Lysine Monohydroch loride 182.6 1.8260 6.47 – 6.54 60 16 L-Proline 115.1 1.1510 6.49 – 6.47 120 9 Supplementary Materials: PANOx-SP Premix Protocols 17 DL-Serine 105.1 1.0510 6.49 – 6.47 30 18 L-Threonine 119.1 1.1910 6.50 – 6.54 30 19 L-Tyrosine 181.2 1.8120 NA NA Table S4: 25x (50 mM) 19 Amino Acid Mix Components. 1. Obtain each of the reagents and set up the stir plate. Place a stir bar into a 300 mL beaker. Add 87.5% (175 mL) of the final solution volume, autoclaved MQ water to the beaker. 2. Place the temperature probe into the water close to the edge of the beaker about half way into the water (without having the probe touching the beaker). Set the heating temperature to 37 °C. Allow the water to reach temperature. Elevated temperatures at even 45 °C for extended time will decrease solution activity. 3. Set the stir rate to 600 rpm. If the final volume is being scaled up, the stir rate should be set so that the bottom of the vortex forms just above the stir bar. 4. Weigh out the required quantity of each of the reagents in Table S4. Add each of the reagents, one at a time according to the order of addition in Table S4. After dissolution of each reagent, measure the pH. Do not add the subsequent reagent until the previous is fully dissolved. Use the “approx. dissolution time” and “approx. pH” as a guide. Repeat this step until tyrosine addition. After threonine has dissolved, allow the solution to mix for an additional 5 minutes. 5. Turn off heating on the heating/stir plate. 6. Pour the solution into a graduated cylinder to determine the volume. Add autoclaved MQ water to bring the total volume to 200 mL (approximately 5% of the final volume will be needed). Sterile filtering the solution may decrease the activity of the final mixture by removing undissolved amino acids. The solution should appear clear and colorless and relatively free of particulates. Freezing the solution at this point should not cause any precipitation. 7. Pour solution back into the beaker and turn on stirring to 600 rpm. While mixing, add the required quantity of tyrosine to the solution and allow the suspension to mix for 5 minutes. Note: the tyrosine will not fully dissolve and will remain in suspension. 8. While still mixing, aliquot 100µL for subsequent reagent testing. 10 Supplementary Materials: PANOx-SP Premix Protocols 9. While still mixing, aliquot the remaining solution into multiple 50-mL conical tubes. Flash freeze the aliquots using liquid nitrogen and store in the -80 °C freezer. 9. Preparation of a 25x PEP Solution (833 mM PEP) (The following protocol describes the preparation of 81.5 mL of 833 mM PEP) Reagent Phosphoenolypyruate (PEP) Formula Weight (g/mol) 206 Desired Concentration in 25x Mix 833 mM Concentration in CFPS Required Mass (g) 33 mM 14 Actual Mass (g) Table S5: 25x PEP Solution Component. 1) Obtain a 200 mL beaker and place it onto a stir plate. Place a stir bar in the beaker. Set up the pH probe by placing it close to the side of the beaker without it touching the walls or the stir bar. 2) Determine the final desired volume for the 25x PEP (81.5 mL). Determine the required amount of PEP for the desired volume (14 g). 3) Add the required quantity of PEP into the beaker. If possible, try to keep the PEP chilled on ice. The PEP should not be left out at room temperature for an extended period of time. 4) For each 1g of PEP used, add 1 mL of water to the beaker. If more than one vial of PEP is used, pour the PEP into the mixing vessel. Then use the 1 mL of water to rinse out any empty bottles of PEP. At this point, the PEP should be mostly insoluble. Increasing the pH will be required to dissolve PEP. 5) Adjust the pH of the PEP solution using 10 M KOH by placing the pH probe into PEP solution. Slowly add 10 M KOH until the pH of the solution reaches pH 7.3 ±0.1 at 22 – 25 °C. The pH increases sharply as the pH nears 7.3 (see attached PEP titration curve, Figure S2). Note: target pH = 7.4. 6) Ensure the complete dissolution of the material before proceeding. 7) Determine the new volume of PEP precursor solution. 8) Determine the volume of the solution using a pipette or graduated cylinder. 11 Supplementary Materials: PANOx-SP Premix Protocols 9) Add autoclaved MQ water to obtain the desired volume of 25x PEP solution. 10) Aliquot 100 µL for subsequent reagent testing. 11) Aliquot the remaining solution into multiple 50-mL conical tubes, flash freeze with liquid nitrogen, and store at -80 °C. PEP Titration (5g PEP / 5mL Water) PEP Titration 8 7 6 pH 5 4 3 2 1 0 0 1000 2000 3000 4000 5000 6000 Volume Titrant (uL) Figure S2: Example PEP titration curve. 10. Testing of individual CFPS precursor solutions All of the above reagents must be first tested at small scale to ensure that all the components are of sufficient quality. Problematic reagents (if any) must be identified and replaced. Component a. Autoclaved MQ Water b. Magnesium Glutamate c. 10x Salt Solution Mix d. 25x Nucleotide Mix e. 25x 19 Amino Acid Mix f. 25x PEP Required Quantity (uL) = 500 – (a + b + c + d + e + f) 20 (For 10 mM) 200 80 80 80 Table S6: Preparation of 500 µL of Test 4x Premix. 1) Thaw the small aliquots (eppendorf tubes) of the precursor solutions. 2) Using the small aliquots, create a scale-down Test 4x Premix solution at 500 µL. This will be used to test the quality of precursor solutions. 12 Supplementary Materials: PANOx-SP Premix Protocols 3) Pipette the required volume of autoclaved MQ water into an eppendorf tube. 4) Add the required volume of the 1000 mM Magnesium Glutamate solution into the eppendorf tube. 5) Add the required volume of the 10x Salt Solution into the eppendorf tube. 6) Add the required volume of the 25x Nucleotide Mix solution into the eppendorf tube. 7) Add the required volume of the 25x 19 Amino Acid Mix into the eppendorf tube. 8) Add the required volume of the 25x PEP solution into the eppendorf tube. 9) Vortex the solution so that it is homogeneous. 10) Perform a set of cell-free reactions using plasmid encoding for chloramphenicol acetyl transferase (CAT) to test reagents. The average total protein yield is between 800-1000 µg/mL. 11) Once the yields have been determined to be sufficient, proceed to the next section of the protocol. If the yields are not sufficient, troubleshooting will be required to determine which components are responsive for the low protein yields. A magnesium optimization may need to be performed for each extract. 11. Combination of individual CFPS precursor solutions into a Final 4x Premix solution Table S7 describes the preparation of the Final 4x PANOx-SP premix. Once the quality of the precursor solutions has been verified using the Test 4x Premix, the large aliquots of the precursor solutions can then be mixed to form the Final 4x Premix. The CFPS yields using the Final 4x Premix should be similar to that of the Test 4x Premix. Component a. Autoclaved MQ Water b. Magnesium Glutamate c. 10x Salt Solution Mix d. 25x Nucleotide Mix e. 25x 19 Amino Acid Mix f. 25x PEP Required Quantity (mL) = 500 – (a + b + c + d + e + f) 20 (For 10 mM) 200 80 80 80 Table S7: Preparation of 500mL of Final 4x Premix. 1) Thaw the large aliquots (50-mL conical tubes) of the precursor solutions. 13 Supplementary Materials: PANOx-SP Premix Protocols 2) Obtain a clean 1000 mL beaker and place it onto a stir plate. Place a stir bar into the bottom of the mixing vessel along with the required amount of autoclaved MQ water. Set the stir rate to a sufficient mixing rate, such that splashing does not occur. 3) Add the required volume of the 1000 mM Magnesium Glutamate solution into the beaker. This value will be dependent on the concentration of magnesium in the cell extract. 4) Add the required volume of the 10x Salt Solution into the beaker. 5) Add the required volume of the 25x Nucleotide Mix solution into the beaker. 6) Add the required volume of the 25x 19 Amino Acid Mix into the beaker. 7) Add the required volume of the 25x PEP solution into the beaker. 8) Increase the stir rate to setting 4. 9) Aliquot the premix into the appropriate 50 µL to 50 mL quantities as desired. 10) Flash freeze the premix aliquots using liquid nitrogen and store at -80 °C. 11) Flash freeze the remaining quantities of 1000 mM Magnesium Glutamate, 10x Salt Solution, 25x Nucleotide Mix, 25x 19 Amino Acid Mix, and 25x PEP solution and store at -80 °C. 12. Conclusions Using the above protocol, we can conveniently produce 500 mL of 4x CFPS premix solution for rapid, convenient, and high yield production of a variety of proteins using cell-free protein synthesis. 14