THE PUBLIC HEALTH ETC - The Scottish Government
advertisement

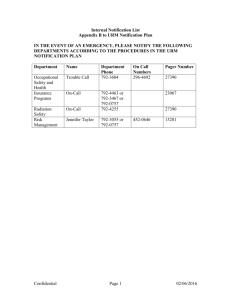
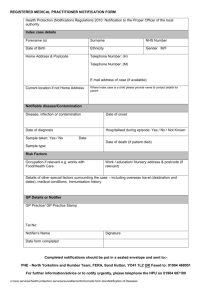
PUBLIC HEALTH ETC. (SCOTLAND) ACT 2008 IMPLEMENTATION OF PART 2: NOTIFIABLE DISEASES, ORGANISMS AND HEALTH RISK STATES Purpose 1. This circular advises that Part 2 and Schedule 1 of the Public Health etc. (Scotland) Act 2008 come into effect on 1 January 2010 and provides supporting guidance to registered medical practitioners, directors of diagnostic laboratories and health boards on their duties under the Act with regard to the notification of infectious diseases, organisms and health risk states. Current responsibilities under the Public Health (Notification of Infectious Diseases) (Scotland) Regulations 1988 and the Infectious Disease (Notification) Act 1889 cease to apply on 31 December 2009. Background 2. The aim of statutory notification is to give early warning of potential threats to human health caused by infectious disease, contamination and other hazards in order to assess what, if any, health protection action might be required to minimise the spread of such diseases and the subsequent risk to human health. The new Act amends the current list of diseases to be notified by registered medical practitioners to health boards (and includes the notification of ‘health risk states’); amends the information to be notified; amends the level of information to be passed on from health boards to Health Protection Scotland (HPS); introduces statutory notification of specific organisms from diagnostic laboratories testing human samples for infection; introduces timescales for notification; and removes the fee to registered medical practitioners for notification. 3. Part 2 and Schedule 1 of the Act, along with further detailed information about the Act and its implementation, can be accessed at http://www.scotland.gov.uk/Topics/Health/NHS-Scotland/publicact Guidance 4. Guidance is contained in the following papers, attached: Paper 1 (and Annexes A & B): Notifiable diseases and health risk states: duties on registered medical practitioners Paper 2 (and Annex C): Notifiable directors of diagnostic laboratories organisms: duties on Paper 3: Notifiable diseases, notifiable organisms and health risk states: duties on health boards Version 1.0 1. November 2009 Action 5. NHS Boards: should bring this Circular to the attention of all registered medical practitioners in their areas, for action, in order to ensure practitioners meet their new duties under the Act (as set out in Paper 1 and Annexes A & B) on 1 January 2010; should bring this Circular to the attention of all diagnostic laboratories in their areas, for action, in order to ensure laboratories meet their new duties under the Act (as set out in Paper 2 and Annex C) on 1 January 2010; should ensure that they are in a position to meet their own statutory obligations (as set out in Paper 3) on 1 January 2010; should also issue the guidance to those registered nurses leading clinics or out of hours services or working in isolated communities without immediate access to a registered medical practitioner. Methods of notification 6. The opportunity is being taken, where possible, to move from paper-based to electronic notification. For registered medical practitioners working in primary care, all notifications with effect from 1 January 2010 will be via the Scottish Care Information (SCI) Gateway. 7. In other settings, paper based arrangements will also be available, as set out in the attached guidance. Version 1.0 2. November 2009 PAPER 1 PUBLIC HEALTH ETC. (SCOTLAND) ACT 2008 NOTIFIABLE DISEASES AND HEALTH RISK STATES: DUTIES ON REGISTERED MEDICAL PRACTITIONERS Registered Medical Practitioners should note that the current arrangements for the notification of infectious diseases will change from 1 January 2010, when Part 2 of The Public Health etc. (Scotland) Act 2008 comes into effect. Current responsibilities under the Public Health (Notification of Infectious Diseases) (Scotland) Regulations 1988 and the Infectious Disease (Notification) Act 1889 cease to apply on 31 December 2009. The new statutory duties are set out below: All registered medical practitioners must notify their health board if they have a reasonable suspicion that a patient whom they are attending has one of the diseases set out in Annex A. Practitioners should not wait until laboratory confirmation of the suspected disease before notification. As well as those diseases which are notifiable in their own right, registered medical practitioners are required to notify any case suffering from a ‘health risk state’ (HRS), and anyone likely to have been exposed to such a case with an HRS, or the same risk factor. Further guidance is contained in Annex B. Please note that we would expect notification of a health risk state to be an exceptional occurrence. Practitioners must notify their health board, in writing (which includes electronic transmission) within 3 days of suspicion, unless he/she has reasonable grounds to believe that another practitioner has notified the disease/HRS. This does not include the potential notification by a laboratory of the organism which causes the disease. Separate notification arrangements are in place for diagnostic laboratories. If the case is ‘urgent’, notification should take place by telephone as soon as reasonably practicable. The need to notify urgently is determined by the registered medical practitioner, having regard to the nature of the disease, the ease of transmission of that disease, the patient’s circumstances and any guidance issued by Scottish Ministers (see overleaf). All urgent oral notifications must be followed up, in writing (which includes electronic transmission), within 3 days of suspicion. For the purposes of electronic notification, a document is to be taken to be received on the day of transmission. Information to be notified (in so far as it is known) the patient’s name; the patient’s address and postcode; the patient’s occupation (if the practitioner considers that it is relevant); the name, address and postcode of the patient’s place of work or education (if the practitioner considers that it is relevant); Version 1.0 3. November 2009 the patient’s sex; the patient’s date of birth; the suspected disease; and the patient’s NHS identifier, i.e. the patient’s community health index number or where that number is not known, the NHS identification number, or where neither of these numbers are known, any other number of other indicators which is used to identify a patient individually. Method of written notification For those registered medical practitioners working in primary care, all written notifications with effect from 1 January 2010 should be undertaken electronically via the Scottish Care Information (SCI) Gateway. A new destination ‘Health Protection Scotland – <NHS board name>’ has been set up on SCI Gateway and a new specialty ‘Notifications reporting’. Registered medical practitioners should use this new destination to inform their local NHS Board Health Protection Team of notifiable disease cases. Enquiries relating to technical issues with SCI Gateway should be addressed to the local SCI Gateway Coordinator. Those registered medical practitioners operating in secondary care who have access to SCI Gateway, are also expected to notify in this way. Where there is no access to SCI Gateway in secondary care, two other methods of notification are available: online – by completing a notification form, available on the Health Protection Scotland (HPS) website at http://www.hps.scot.nhs.uk/publichealthact/index.aspx and sending it by secure e-mail, within the time limits set out in this guidance, to the Health Protection Team in the Health Board. for those without ready access to a PC – by completing a paper copy of the above form and sending in a secure manner, or sending by secure fax to the Health Protection Team in the Health Board. Local contacts The Act requires notification to the health board for the area in which the practitioner works, in effect the local Health Protection Team. Contact details of health boards’ Health Protection Teams are set out in Annex D. Guidance It is recommended that those diseases marked with an * in Annex A require urgent notification, i.e. within same working day. However, there may be other circumstances where immediate notification might be necessary, e.g. if there are a cluster of cases. Further guidance on the need for telephone notification may be provided locally from time to time, based on changing epidemiology or particular local circumstances. If in any doubt, call the Health Protection Team at your local health board. Version 1.0 4. November 2009 It is recognised that there may be nurses leading clinics or out of hours services, or working in isolated communities who may well form a suspicion that a patient has a notifiable disease which requires immediate action. If such a nurse were to communicate a suspicion by telephone to a registered medical practitioner, the practitioner may choose to commence notification procedures immediately on this basis. Should there be delay in contacting a registered medical practitioner to facilitate notification, for any reason, the registered nurse should call the health protection team at the local health board directly to inform them of their suspicion. However, this does not constitute a formal notification under the Act. The registered nurse should continue to pursue formal notification through the relevant registered medical practitioner. Nurses should ensure that they clearly document all action taken and advice given when raising a suspicion. Registered medical practitioners should not attempt to ‘denotify’ when the laboratory results indicate that the patient does not have the suspected notifiable disease. The Health Protection Team should, however, be contacted if an administrative error has been made in the notification process. Where a patient presents with a notifiable disease for the first time in Scotland and the patient has already been diagnosed with that condition in another part of the UK, or abroad, registered medical practitioners should notify. Once statutory notification is made, it would be helpful if the notification is noted in the patient’s records. This should help to avoid duplicate notifications. Further information Notifications will be considerably reduced from pre-2010 levels because the list only contains diseases which may prompt urgent public health investigation and action. For example, food poisoning and chickenpox, which constituted about 80% of notifications prior to 1 January 2010, have been removed. The fee to registered medical practitioners has been removed, recognising that notification of the significant diseases on the new list will become an unusual event for a medical practitioner and should be undertaken under his/her general duty of care to protect public health. Non-notification is a governance issue which falls to the health board for employed doctors, and for GPs as independent contractors, through their own clinical governance systems, and ultimately through the GMS contractual requirement in Schedule 5, paragraph 115 of The NHS (General Medical Services Contracts) (Scotland) Regulations 2004 and Schedule 1, paragraph 79 of The NHS (Primary Medical Services Section 17C Agreements)(Scotland) Regulations 2004, i.e. the contractor shall comply with all relevant legislation; and have regard to all relevant guidance issued by the health board and the Scottish Ministers. Scottish Ministers may amend the list of diseases to be notified, by regulation, by adding to or removing an item from the list, and varying the description of an item in the list. Scottish Ministers may also, by regulation, amend any other aspect of the notification arrangements. Version 1.0 5. November 2009 Version 1.0 6. November 2009 PAPER 2 PUBLIC HEALTH ETC. (SCOTLAND) ACT 2008 NOTIFIABLE ORGANISMS: LABORATORIES DUTIES ON DIRECTORS OF DIAGNOSTIC Directors of diagnostic laboratories in Scotland, as defined overleaf, should note the new statutory notification duties being placed on them by The Public Health etc. (Scotland) Act 2008, with effect from 1 January 2010. The director of a diagnostic laboratory in Scotland must notify the organisms set out in Annex C to the health board in whose area the laboratory is situated and Health Protection Scotland (HPS). Notification must take place, in writing, within 10 days of identification. Electronic notification, e.g. through Electronic Communication of Surveillance in Scotland (ECOSS), is acceptable. For the purposes of electronic notification, a document is to be taken to be received on the day of transmission. If the case is ‘urgent’, notification should take place by telephone as soon as reasonably practicable. ‘Urgency’ is determined by the director of the laboratory, having regard to the nature of the organism, the nature of the disease caused by the organism, the ease of transmission, the patient’s circumstances (where known) and any guidance issued by Scottish Ministers. See guidance below. All urgent notifications require to be followed up in writing within 10 days of identification. Where a diagnostic laboratory requires, under an arrangement, to send a sample to another laboratory for analysis (e.g. to a specialist facility in Scotland or elsewhere in the UK), statutory notification is required from the originating laboratory. In these cases, the day of identification for the purposes of notification will be the day on which the first diagnostic laboratory becomes aware of the identification by the other laboratory with which it has the arrangement. Information to be notified (in so far as it is known) the name of the person to whom the identification relates; the person’s address; the person’s sex; the person’s date of birth; the organism which has been identified; and the person’s NHS identifier, i.e. the patient’s community health index number or, where that number is not known, the NHS identification number, or where neither of these numbers are known, any other number or other indicator which is used to identify a patient individually. Version 1.0 7. November 2009 Method of notification The existing ECOSS reporting system, already in use at most NHS diagnostic laboratories, meets the new statutory notification requirements, and should continue to be used from 1 January 2010. An e-mail alert will be sent to the Health Protection Team of the local health board when an electronic Weekly Report is available. Those laboratories not currently using ECOSS should continue with existing methods of notification until such time as ECOSS is implemented. In the case of private laboratories, an automated electronic reporting system will be implemented, where possible. However, in the short term, private laboratories should send a secure electronic notification via email or secure fax to the health board in whose area the laboratory is situated and to HPS within the time limits specified in the Act and as set out in this guidance. Local contacts The Act requires notification to the health board in whose area the diagnostic laboratory is situated, in effect the local Health Protection Team. Contact details of health boards’ Health Protection Teams are set out in Annex D. Guidance It is recommended that those organisms marked with an * in Annex C require urgent notification, i.e. within the same working day. There may be circumstances where notification well within the 10 day period would be appropriate, but might not necessarily merit urgent telephone notification, or where organisms might need to be notified to health boards before formal identification. Laboratories should therefore ensure that they have mutually agreed Standard Operating Procedures or other agreements in place with local health boards, which clarify these issues and take into account their new statutory obligations. These agreements may also cover emerging organisms which have public health consequences. ‘Diagnostic laboratory’ means an institution (or facility within an institution) which is equipped with apparatus and reagents for the performance of diagnostic tests for human infections. ‘Director of a diagnostic laboratory’ means – - the clinical microbiologist, consultant pathologist or other registered medical practitioner or other person in charge of a diagnostic laboratory; or - any other person working in the diagnostic laboratory to whom the function of making a notification under this section has been delegated by the person mentioned above. Offences It is an offence for the director of a diagnostic laboratory to fail, without reasonable excuse, to comply with the duty of notification. Where the director of a diagnostic laboratory commits an offence and is employed by a body corporate, the body corporate also commits the offence. There is a defence Version 1.0 8. November 2009 of due diligence and that all reasonable steps were taken to avoid committing the offence, both for a director of the diagnostic laboratory and a body corporate, or its employee or agent. Further information Scottish Ministers may amend the list of organisms to be notified, by regulation, by adding to or removing an item from the list, and varying the description of an item on the list. Scottish Ministers may also, by regulation, amend any aspect of the notification arrangements. Version 1.0 9. November 2009 Version 1.0 10. November 2009 PAPER 3 PUBLIC HEALTH ETC. (SCOTLAND) ACT 2008 NOTIFIABLE DISEASES, NOTIFIABLE ORGANISMS AND HEALTH RISK STATES: DUTIES ON HEALTH BOARDS Where a health board receives a notification of a disease or health risk state from a registered medical practitioner and the patient to whom the information relates usually resides in that health board area, the board must send a return, in writing (in practice electronic transmission via Scottish Infectious Disease Surveillance System (SIDSS2), to Health Protection Scotland (HPS), containing the information set out below. The return should be sent by the end of the week in which the information is received (in the Act ‘week’ is defined as meaning a period of 7 days ending on Friday at the expiry of the normal working hours of the board’s principal office), or, if it is not practicable to do so, as soon as practicable afterwards. For the purposes of electronic transmission of data, a document is to be taken to be received on the day of transmission. If the patient to whom the information relates does not usually reside in that health board area, the board must without delay transmit the information to the health board for the area in which the person usually resides. Where a health board receives information from another health board about a patient who usually resides in its area, that board must send the return, in writing, to HPS, by the end of the working week in which it is received (i.e. by close of play on Friday of that week) or, if it is not practicable to do so, as soon as practicable afterwards. Where a health board receives information from a laboratory and that information relates to a person who does not usually reside in its area, the board must, without delay, transmit that information to the health board for the area in which the person usually resides. Information to be included in return to HPS (in so far as it is known) the patient’s postcode; the patient’s occupation; the patient’s sex; the patient’s date of birth; the suspected disease or suspected health risk state; and the patient’s NHS identifier, i.e. the patient’s community health index number or where that number is not known, the NHS identification number, or where neither of these numbers are known, any other number or other indicator which is used to identify a patient individually. Method of notification SIDSS2 will continue to be the means of electronic notification from the health board to HPS. A number of changes have been made to the input screen to reflect the information that now requires to be captured to meet the requirements of the Act. Version 1.0 11. November 2009 Version 1.0 12. November 2009 ANNEX A DISEASES TO BE NOTIFIED BY REGISTERED MEDICAL PRACTITIONERS WITH EFFECT FROM 1 JANUARY 2010: NOTIFICATIONS ARE BASED ON REASONABLE SUSPICION AND SHOULD NOT AWAIT LAB. CONFIRMATION * Anthrax * Botulism Brucellosis * Cholera * Clinical syndrome due to E.coli O157 infection (see Note 1) * Diphtheria * Haemolytic Uraemic Syndrome (HUS) * Haemophilus influenzae type b (Hib) * Measles * Meningococcal disease Mumps * Necrotizing fasciitis * Paratyphoid * Pertussis * Plague * Poliomyelitis * Rabies Rubella * Severe Acute Respiratory Syndrome (SARS) * Smallpox Tetanus Tuberculosis (respiratory or non-respiratory) (see Note 2) * Tularemia * Typhoid * Viral haemorrhagic fevers * West Nile fever Yellow Fever *It is recommended that those diseases above marked with an * require urgent notification, i.e. within the same working day. Follow up written / electronic notification within 3 days is still required. Note 1: E.coli O157 Clinical suspicion should be aroused by (i) likely infectious bloody diarrhoea or (ii) acute onset non-bloody diarrhoea with a biologically plausible exposure and no alternative explanation. Examples of biologically plausible exposures include: contact with farm animals, their faeces or environment; drinking privately supplied or raw water; eating foods such as undercooked burgers or unpasteurised dairy products; contact with a confirmed or suspected case of VTEC infection. Further guidance is available at: http://www.hps.scot.nhs.uk/giz/e.coli0157.aspx?subjectid=18 Cases notified as HUS (Haemolytic Uraemic Syndrome) should NOT be notified as “Clinical syndrome due to E.coli O157 infection” as well. Note 2: Tuberculosis For the purposes of notification, respiratory TB or non-respiratory TB should be taken to have the same meanings as the World Health Organisation definitions of pulmonary TB and non-pulmonary TB respectively. Pulmonary TB is tuberculosis of the lung parenchyma and/or the tracheobronchial tree. Non-pulmonary TB is tuberculosis of any other site. Where tuberculosis is clinically diagnosed in both pulmonary and non-pulmonary sites, this should be treated as pulmonary TB. If you are in any doubt about the diagnosis of suspected cases, you should contact the local Health Protection Team for advice (Annex D). Version 1.0 13. November 2009 Version 1.0 14. November 2009 ANNEX B NOTIFICATION OF HEALTH RISK STATES (HRS) Why is it necessary to notify suspected 'health risk states'? The aim of notifying suspected health risk states is to identify diseases or conditions which are not notifiable in their own right but which pose or may pose a significant risk to public health. The definition of ‘health risk state’ and ‘exposure to a health risk state’, as provided in the Act is set out in the footnote1. Pubic health authorities need to be able to identify and respond quickly to new and emerging public health threats, even when a condition is identified from its symptoms and epidemiology and the causative organism is not yet identified. This is particularly relevant in the modern world of global travel and trade. For example, urgent public health action was required in the early stages of the SARS (Severe Acute Respiratory Syndrome) outbreak in 2003 and the Influenza AH1N1 outbreak in 2009, even before the causative agent was known. Other examples from the past of conditions that would fulfil the criteria of a health risk state include the initial five cases of Pneumocystis carinii pneumonia heralding the AIDS epidemic, avian flu, Polonium exposure and poisoning in the Litvinenko case. What should be notified? This is for practitioners to determine, based on reasonable suspicion. However, the following advice should assist. (a) If a novel serious condition occurs at home or abroad, it may be designated an HRS by the Scottish Government's Chief Medical Officer (CMO), who will provide a case definition for exactly what should be notified. (b) In the absence of a definition from the CMO, medical practitioners should notify as an HRS any condition which is: 1. Serious: A case must be very ill or have died, or be likely to become very ill or die. AND 2. Be potentially serious to others. 1 Section 14 (7) of the Public Health etc. (Scotland) Act 2008 defines a ‘health risk state’ as (a) a highly pathogenic infection; or (b) any contamination, poison or other hazard which is a significant risk to public health. References to a patient having been ‘exposed to a health risk state’ is defined in section 14 (8) as (a) having been in physical contact with a health risk state; (b) having been contaminated by a health risk state; or (c) having been in physical contact with or contaminated by a person who, or an object which, has been in physical contact with or contaminated by a health risk state. Version 1.0 15. November 2009 The three principal ways in which an HRS might be serious for others are if it is: (i) infectious; (ii) the result of contamination with, for example, a radioactive material; (iii) the result of a toxin or poison to which others may be exposed. An HRS is likely to be new, rare, unexplained, or difficult to diagnose. Obviously, the more serious the condition, and the greater the likelihood of spread, the more important it is for the medical practitioner to notify it. The more cases the medical practitioner sees within a given period of time, the more likely is the potential for spread, but an HRS presenting in a single case may still have the potential to affect others. Notifications of an HRS will be an exceptional occurrence and should only be made on the basis that the registered medical practitioner considers there is a risk of significant public health implications of the condition. If in doubt whether to notify a condition, on grounds of either its seriousness or potential to affect others, the medical practitioner should discuss the condition with the local Health Protection Team (Annex D). How to notify Immediate oral notification by telephone is strongly recommended in these circumstances, followed up in writing / electronically with the required information within 3 days of forming the suspicion of the health risk state. Version 1.0 16. November 2009 ANNEX C NOTIFIABLE ORGANISMS LABORATORIES FOR NOTIFICATION BY DIAGNOSTIC *Bacillus anthracis Bacillus cereus *Bordetella pertussis Borrelia burgdorferi Brucella genus *Campylobacter genus Chlamydia psittaci *Clostridium botulinum Clostridium difficile Clostridium perfringens *Clostridium tetani *Corynebacterium diphtheriae (toxigenic strains) *Corynebacterium ulcerans *Coxiella burnetii *Crimean-Congo haemorrhagic fever virus *Cryptosporidium Dengue virus *Ebola virus Echinococcus genus *Verocytotoxin-producing E.coli (VTEC) *Francisella tularensis *Giardia lamblia *Guanarito virus *Haemophilus influenzae type b (from blood, cerebrospinal fluid or other normally sterile site) Hantavirus *Hepatitis A virus *Hepatitis B virus (see Note 1) Hepatitis C virus Hepatitis E virus Version 1.0 17. November 2009 Influenza virus (all types, including *those caused by a new sub-type) *Junín virus *Kyasanur Forest disease virus *Lassa virus *Legionella genus Leptospira genus *Listeria monocytogenes *Machupo virus *Marburg virus *Measles virus Mumps virus *Mycobacterium bovis *Mycobacterium tuberculosis complex *Neisseria meningitidis Norovirus *Omsk haemorrhagic fever virus Plasmodium falciparum, vivax, ovale and malariae *Polio virus *Rabies virus Rickettsia prowazekii *Rift Valley fever virus *Rubella virus *Sabia virus *Salmonella (all human types) *SARS-associated coronavirus *Shigella genus Enterotoxigenic Staphylococcus aureus Staphylococcus aureus (all blood isolates) Methicillin-resistant Staphylococcus aureus (MRSA) *Streptococcus pyogenes (from blood, cerebrospinal fluid or other normally sterile site) Streptococcus pneumoniae (from blood, cerebrospinal fluid or other normally sterile site) Version 1.0 18. November 2009 Toxoplasma gondii. Trichinella genus Varicella-zoster virus *Variola virus *Vibrio cholerae *West Nile fever virus *Yellow Fever virus *Yersinia enterocolitica *Yersinia pestis *Yersinia pseudotuberculosis *It is recommended that those organisms above marked with an * require urgent notification, i.e. within the same working day. Follow up written / electronic notification within 10 days is still required. Note 1: For Hepatitis B, only acute infections require urgent notification. Version 1.0 19. November 2009 Version 1.0 20. November 2009 ANNEX D HEALTH PROTECTION TEAM CONTACTS IN NHS BOARDS NHS Ayrshire and Arran Tel: 01292 611040 Tel: 01563 521133 (Out of Hours) Fax: 01292 885902 E-mail: HPTeam@aapct.scot.nhs.uk NHS Highland Tel: 01463 704886 Tel: 01463 704000 (Out of Hours) Fax: 01463 717666 E-mail: iris.mackenzie@nhs.net NHS Borders Tel: 01896 825560 Tel: 01896 826000 (Out of Hours) Fax: 01896 823396 E-mail: tim.patterson@borders.scot.nhs.uk NHS Lanarkshire Tel: 01698 206326 Tel: 01236 748748 (Out of Hours) Fax: 01698 424316 E-mail: healthprotection@lanarkshire.scot.nhs.uk NHS Dumfries and Galloway Tel: 01387 272724 Tel: 01387 246246 (Out of Hours) Fax: 01387 272759 E-mail: dumf-uhb.hpt@nhs.net NHS Lothian Tel: 0131 536 9192/9092 Tel: 0131 242 1000 (Out of Hours) Fax: 0131 536 9195 E-mail: health.protection@nhslothian.scot.nhs.uk. NHS Fife Tel: 01592 226435 Tel: 01383 623623 (Out of Hours) Fax: 01592 226925 E-mail: hpt.fife@nhs.net NHS Shetland Tel: 01595 743072 Tel: 01595 743000 (Out of Hours) Fax: 01595 695200 E-mail: shet-hb.PublicHealthShetland@nhs.net NHS Forth Valley Tel: 01786 457283 Tel: 01786 434000 (Out of Hours) Fax: 01786 446327 E-mail: henry.prempeh@nhs.net NHS Tayside Tel: 01382 596976/87 Tel: 01382 660111 (Out of Hours) Fax: 01382 596985 E-mail: healthprotectionteam@tayside.nhs.net NHS Grampian and NHS Orkney Tel: 01224 558520 Tel: 0845 456 6000 (Out of Hours) Fax: 01224 558566 E-mail: grampian.healthprotection@nhs.net NHS Western Isles Tel: 01851 708033 Tel: 01851 704704 (Out of Hours) Fax: 01851 702036 E-mail: angelagrant1@nhs.net NHS Greater Glasgow and Clyde Tel: 0141 201 4917 Tel: 0141 211 3600 (Out of Hours) Fax: 0141 201 4950 E-mail: PHPU@ggc.scot.nhs.uk Version 1.0 21. November 2009