POLA_25041_sm_SuppInfo1
advertisement

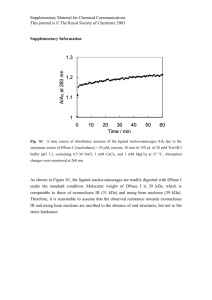
High Molecular Weight Polyacrylamides by ATRP: Enabling Advancements in Water-Based Applications Eric A. Appel, Jesús del Barrio, Xian Jun Loh, Joseph Dyson, and Oren A. Scherman Melville Laboratory for Polymer Synthesis, Department of Chemistry, University of Cambridge, Lensfield Road, Cambridge CB2 1EW, United Kingdom S.1 Instrumentation and Materials 1 H NMR (400 MHz) spectra was recorded using a Bruker Avance QNP 400. Chemical shifts are recorded in ppm (δ ) in D2 O with the internal reference set to δ 4.79 ppm. Gel permeation chromatography (GPC) was carried out in either tetrahydrofuran (THF) or in water (H2 O). Aqueous GPC was performed on a Shodex glucose column with a Shimadzu SPD-M20A prominence diode array detector, Optilab refractive index detector and dynamic light scattering detector (both Wyatt). Samples were filtered over 0.2 µ m PVDF filters before injection using a 0.6 mL / min flow rate. THF GPC was performed on two Jordi 5µ m DVB columns connected in series with a SPD-M20A prominence diode array detector and refractive index detector (both Shimadzu) calibrated in relation to poly(methyl methacrylate) standards. Samples were filtered over 0.45 µ m PTFE filters before injection using a 1.0 mL / min flow rate. N, N’-dimethylacrylamide (DMAm), N-hydroxyethylacrylamide (HEAm) was purchased from Aldrich and purified on a basic alumina column to remove the inhibitor and stored over 4Å molecular sieves. Nisopropylacrylamide (NIPAm) was purchased from Aldrich and recrystallized from a mixture of toluene and hexane. All other materials were purchased from Sigma-Aldrich and used as received. S.2 General Synthetic Protocols General synthesis of Poly(N-isopropylacrylamide). N-Isopropylacrylamide (0.49 g, 4.3 mmol) and methyl 2-chloropropanoate (MCP) (1.1 mg, 0.98 µ L, 8.7 µ mol, Target DP = [M]/[I] = 500) were dissolved in a 1:4 (v/v) mixture of deionized water and ethanol (0.75 mL) and the solution was degassed by bubbling nitrogen for 5 min. CuCl (0.9 mg, 8.7 µ mol) and Me6 TREN (2.0 mg, 2.4 µ L, 8.7 µ mol) were dissolved in a 1:4 (v/v) mixture of deionized water and ethanol (0.25 mL) and the solution was degassed by bubbling nitrogen for 5 min. The monomer and initiator solution was then added via syringe into the copper and ligand solution and the mixture was allowed to stir at 20 o C for 30 min. Aliquots were removed periodically to analyze conversion. The reaction mixture was then diluted with ethanol (5 mL) and precipitated into cold diethyl ether. The resulting residue was redissolved in chloroform, dried over magnesium sulfate, filtered and precipitated from cold diethyl ether. The solid was filtered and dried under vacuum yielding the title compound as a white solid (0.45 g, 93 %). 1 H-NMR Spectroscopy (D2 O, 400 MHz) δ (ppm) = 3.67-3.50 (1H, (CH3 )2 -CH-NH-), 2.2-0.8 (polymer backbone and (CH3 )2 -CH-NH-). GPC (H2 O): Mn (PDI) = 56.0 kDa (1.06). S1 (a) (b) PDI PDI PDI Mn (kDa) Mn PDI Mn (kDa) Mn [M]o/[I]o Conversion (%) Figure S1: (a) Mn and PDI versus conversion for the CuCl/Me6 -TREN catalyzed SET-LRP of Nisopropylacylamide (NIPAm) with [NIPAm]o :[MCP]o :[CuCl]o :[Me6 -TREN]o = 500:1:1:1. (b) Plot demonstrating the effect of [NIPAm]o /[MCP]o on Mn and PDI for the polymerization of NIPAm. Table S1: Polymerization of N-isopropylacrylamide using the CuCl/Me6 -TREN catalyst system. Entry 1 2 3 2 [M]o /[I]o Solvent (EtOH:H2 O) Mn (kDa)a 50 50:50 17 50 70:30 14 100 50:50 16 1000 50:50 192 a determined by GPC using H2 O as eluent. 100 2 Mn from GPC (kDa) PDI 50 1.4 1.5 1.3 PDI 1 40 40 Mn (kDa) 60 Ln([M]o/[M]) Conversion (%) 1.5 60 Conversion Ln([M]o/[M]) 80 PDIa 1.10 1.17 1.08 1.22 30 1.2 20 0.5 20 1.1 10 kp = 0.0201 min-1 0 0 10 20 30 40 50 60 Ieff = 98% 0 0 70 0 10 20 30 40 50 1 Mn Theoretical (kDa) Time (min) Figure S2: (a) Conversion and ln([M]o /[M]) versus time for the CuCl/Me6 -TREN catalyzed SET-LRP of Nisopropylacylamide (NIPAm) with [NIPAm]o :[MCP]o :[CuCl]o :[Me6 -TREN]o = 500:1:1:1. (b) Plot demonstrating the effect of initiator efficiency for the polymerization of NIPAm. S2 General synthesis of Poly(N, N’-Dimethylacrylamide). N, N’-Dimethylacrylamide (0.43 g, 4.3 mmol) and methyl 2-chloropropanoate (MCP) (1.1 mg, 0.98 µ L, 8.7 µ mol, Target DP = [M]/[I] = 500) were dissolved in a 1:4 (v/v) mixture of deionized water and ethanol (0.75 mL) and the solution was degassed by bubbling nitrogen for 5 min. CuCl (0.9 mg, 8.7 µ mol) and Me6 TREN (2.0 mg, 2.4 µ L, 8.7 µ mol) were dissolved in a 1:4 (v/v) mixture of deionized water and ethanol (0.25 mL) and the solution was degassed by bubbling nitrogen for 5 min. The monomer and initiator solution was then added via syringe into the copper and ligand solution and the mixture was allowed to stir at 20 o C for 30 min. Aliquots were removed periodically to analyze conversion. The reaction mixture was then diluted with ethanol (5 mL) and precipitated into cold diethyl ether. The resulting residue was redissolved in chloroform, dried over magnesium sulfate, filtered and precipitated from cold diethyl ether. The solid was filtered and dried under vacuum yielding the title compound as a white solid (0.40 g, 94 %). 1 H-NMR Spectroscopy (D2 O, 400 MHz) δ (ppm) = 3.50-3.45 (6H, (CH3 )2 N-COO-CH-CH2 ), 2.45-2.00 (1H, (CH3 )2 N-COO-CH-CH2 ), 1.95-1.35 (2H, H2 N-COO-CHCH2 ). GPC (H2 O): Mn (PDI) = 40.6 kDa (1.08). (a) (b) Mn Mn PDI PDI Mn (kDa) PDI Mn (kDa) PDI [M]o/[I]o Conversion (%) Figure S3: (a) Mn and PDI versus conversion for the CuCl/Me6 -TREN catalyzed SET-LRP of N, N’dimethylacrylamide (DMAm) with [DMA]o :[MCP]o :[CuCl]o :[Me6 -TREN]o = 500:1:1:1. (b) Plot demonstrating the effect of [DMAm]o /[MCP]o on Mn and PDI for the polymerization of DMAm. S3 1.6 60 Conversion Ln([M]o/[M]) Mn from GPC (kDa) PDI 1.4 40 1.4 1 30 0.8 0.6 20 0.4 10 30 1.3 20 1.2 PDI 40 Mn (kDa) 1.2 Ln([M]o/[M]) Conversion (%) 50 1.5 50 10 0.2 kp = 0.0474 min-1 0 0 5 10 15 0 0 1.1 Ieff = 78% 0 20 5 10 15 20 25 30 1 Mn Theoretical (kDa) Time (min) Figure S4: (a) Conversion and ln([M]o /[M]) versus time for the CuCl/Me6 -TREN catalyzed SET-LRP of N, N’-dimethylacylamide (DMAm) with [DMAm]o :[MCP]o :[CuCl]o :[Me6 -TREN]o = 500:1:1:1. (b) Plot demonstrating the effect of initiator efficiency for the polymerization of DMAm. S4 General synthesis of Poly(N-hydroxyethylacrylamide). N-Hydroxyethylacrylamide (0.5 g, 4.3 mmol) and methyl 2-chloropropanoate (MCP) (1.1 mg, 0.98 µ L, 8.7 µ mol, Target DP = [M]/[I] = 500) were dissolved in a 1:1 (v/v) mixture of deionized water and ethanol (0.75 mL) and the solution was degassed by bubbling nitrogen for 5 min. CuCl (0.9 mg, 8.7 µ mol) and Me6 TREN (2.0 mg, 2.4 µ L, 8.7 µ mol) were dissolved in a 1:1 (v/v) mixture of deionized water and ethanol (0.25 mL) and the solution was degassed by bubbling nitrogen for 5 min. The monomer and initiator solution was then added via syringe into the copper and ligand solution and the mixture was allowed to stir at 20 o C for 30 min. Aliquots were removed periodically to analyze conversion. The reaction mixture was then diluted with ethanol (5 mL) and precipitated into acetone. The solid was filtered and dried under vacuum yielding the title compound as a white solid (0.48 g, 96 %). 1 H-NMR Spectroscopy (D2 O, 400 MHz) δ (ppm) = 3.50-3.32 (2H, HO-CH2 -CH2 ), 3.25-2.87 (2H, HEAm, HO-CH2 -CH2 ), 2.2-0.8 (polymer backbone). GPC (H2 O): Mn (PDI) = 59.2 kDa (1.10). (b) PDI PDI Mn (kDa) Mn Mn (kDa) Mn PDI PDI (a) [M]o/[I]o Conversion (%) Figure S5: (a) Mn and PDI versus conversion for the CuCl/Me6 -TREN catalyzed SET-LRP of Nhydroxyethylacylamide (HEAm) with [HEAm]o :[MCP]o :[CuCl]o :[Me6 -TREN]o = 500:1:1:1. (b) Plot demonstrating the effect of [HEAm]o /[MCP]o on Mn and PDI for the polymerization of HEAm. Table S2: Polymerization of N-hydroxyethylacrylamide using the CuCl/Me6 -TREN catalyst system. Entry 1 2 a [M]o /[I]o Solvent (EtOH:H2 O) Conversion (%)a Mn (kDa)b PDIb 5000 80:20 36 180 1.68 5000 30:70 85 405 1.75 1 b determined by H NMR. determined by GPC using H2 O as eluent. S5 Mn from GPC (kDa) PDI 50 0.8 1.4 40 0.4 20 0.2 10 kp = 0.1537 min 0 0 1 2 3 4 1.3 PDI 30 40 Mn (kDa) 0.6 Ln([M]o/[M]) Conversion (%) 50 1.5 60 1 Conversion Ln([M]o/[M]) 30 1.2 20 1.1 10 -1 0 0 5 Ieff = 82% 1 0 Time (min) 10 20 30 40 50 Mn Theoretical (kDa) Figure S6: (a) Conversion and ln([M]o /[M]) versus time for the CuCl/Me6 -TREN catalyzed SET-LRP of N-hydroxyethylacylamide (HEAm) with [HEAm]o :[MCP]o :[CuCl]o :[Me6 -TREN]o = 500:1:1:1. (b) Plot demonstrating the effect of initiator efficiency for the polymerization of HEAm. S6 General synthesis of Poly(acrylamide). Acrylamide (0.31 g, 4.3 mmol) and methyl 2-chloropropanoate (MCP) (1.1 mg, 0.98 µ L, 8.7 µ mol, Target DP = [M]/[I] = 500) were dissolved in a 7:3 (v/v) mixture of deionized water and ethanol (0.75 mL) and the solution was degassed by bubbling nitrogen for 5 min. CuCl (0.9 mg, 8.7 µ mol) and Me6 TREN (2.0 mg, 2.4 µ L, 8.7 µ mol) were dissolved in a 7:3 (v/v) mixture of deionized water and ethanol (0.25 mL) and the solution was degassed by bubbling nitrogen for 5 min. The monomer and initiator solution was then added via syringe into the copper and ligand solution and the mixture was allowed to stir at 20 o C for 30 min. Aliquots were removed periodically to analyze conversion. The reaction mixture was then diluted with ethanol (5 mL) and precipitated into acetone. The solid was filtered and dried under vacuum yielding the title compound as a white solid (0.29 g, 95 %). 1 H-NMR Spectroscopy (D2 O, 400 MHz) δ (ppm) = 2.45-2.00 (H2 N-COO-CH-CH2 ), 1.95-1.35 (H2 N-COO-CH-CH2 ). GPC (DMF): Mn (PDI) = 47.9 kDa (1.16). (a) (b) Mn Mn PDI Conversion (%) PDI Mn (kDa) PDI Mn (kDa) PDI [M]o/[I]o Figure S7: (a) Mn and PDI versus conversion for the CuCl/Me6 -TREN catalyzed SET-LRP of acylamide (Am) with [Am]o :[MCP]o :[CuCl]o :[Me6 -TREN]o = 500:1:1:1. (b) Plot demonstrating the effect of [Am]o /[MCP]o on Mn and PDI for the polymerization of Am. S7 30 50 40 Mn from GPC (kDa) PDI 25 1.4 0.8 0.4 20 20 1.3 PDI 0.6 Mn (kDa) 30 Ln([M]o/[M]) Conversion (%) 1.5 1 Conversion Ln([M]o/[M]) 15 1.2 10 0.2 10 1.1 5 kp = 0.1298 min 0 0 0 1 2 3 4 Ieff = 80% -1 0 5 1 0 Time (min) 5 10 15 20 Mn Theoretical (kDa) Figure S8: (a) Conversion and ln([M]o /[M]) versus time for the CuCl/Me6 -TREN catalyzed SET-LRP of acylamide (Am) with [Am]o :[MCP]o :[CuCl]o :[Me6 -TREN]o = 500:1:1:1. (b) Plot demonstrating the effect of initiator efficiency for the polymerization of Am. S8 General synthesis of Poly(NIPAM-r-HEAm) 500:500. N-Isopropylacrylamide (0.49 g, 4.3 mmol), Nhydroxyethylacrylamide (0.5 g, 4.3 mmol), and methyl 2-chloropropanoate (MCP) (1.1 mg, 0.98 µ L, 8.7 µ mol, total DP=[M]/[I]=1000) were dissolved in a 1:1 (v/v) mixture of deionized water and ethanol (2 mL) and the solution was degassed by bubbling nitrogen for 5 min. CuCl (0.9 mg, 8.7 µ mol) and Me6 TREN (2.0 mg, 2.4 µ L, 8.7 µ mol) were dissolved in a 1:4 (v/v) mixture of deionized water and ethanol (1.0 mL) and the solution was degassed by bubbling nitrogen for 5 min. The monomer and initiator solution was then added via syringe into the copper and ligand solution and the mixture was allowed to stir at 20 o C for 30 min. Aliquots were removed periodically to analyze conversion. The reaction mixture was then diluted with ethanol (5 mL) and precipitated into cold acetone. The solid was filtered and dried under vacuum yielding the title compound as a white solid (0.40 g, 94 %). The composition of the final copolymers was determined by 1 H-NMR using integration of the signals for the single proton α to the amide functionality in NIPAm and the two protons α to the hydroxyl functionality in HEAm (Figure S9 in the supporting information). 1 H-NMR Spectroscopy (D2 O, 400 MHz) δ (ppm) = 3.67-3.50 (1H, NIPAm, (CH3 )2 -CH-NH), 3.50-3.32 (2.2H, HEAm, HO-CH2 -CH2 ), 3.25-2.87 (2.2H, HEAm, HO-CH2 -CH2 ), 2.2-0.8 (HEAm and NIPAm polymer backbone and NIPAm (CH3 )2 -CH-NH-)). GPC (H2 O): Mn (PDI) = 66.0 kDa (1.16). 3.0 2.5 2.0 1.5 1.0 0.5 ppm 2.2 3.5 2.2 4.0 1.0 4.5 Figure S9: 1 H-NMR spectrum of poly(NIPAM-r-HEAm) (500:500) in D2 O. S9 Table S3: Random copolymerization of acrylamides initiated with MCP and using the CuCl/Me6 -TREN catalyst system. Entry Monomer Aa Monomer Ba Target B (%) Experimental B (%)b Mn (kDa)c PDIc 1d NIPAm DMAm 10 17.7 85 1.22 NIPAm DMAm 20 32.0 66 1.17 2d e NIPAm HEAm 10 12.7 56 1.15 3 NIPAm HEAm 20 22.2 47 1.18 4e e NIPAm HEAm 30 31.7 56 1.17 5 e NIPAm HEAm 50 52.4 66 1.16 6 HEAm Am 50 52.5 47 1.16 7e a NIPAm = N-isopropylacrylamide, HEAm = N-hydroxyethylacrylamide, DMAm = N, N’-dimethylacrylamide and Am = acrylamide. b determined by 1 H NMR. c determined by GPC using H2 O as eluent. d [M]o :[MCP]o :[CuCl]o :[Me6 -TREN]o = 1000:1:1:1 e [M]o :[MCP]o :[CuCl]o :[Me6 -TREN]o = 500:1:1:1 S10 S.3 Characterization of Poly(N-Isopropylacrylamide) Thermoresponsive Behavior Figure S10: Plot demonstrating the effect of poly(NIPAm) molecular weight on the observed LCST for both heating and cooling cycles of the polymers in water. The legend refers to the DP of the poly(NIPAm) polymers. Figure S11: Plot demonstrating the effect of poly(NIPAm) molecular weight on the observed difference in LCST between heating and cooling of the polymers in water. The plot demonstrates a logarithmic relationship between the Mn in kDa and the change in LCST according to the following equation: ∆LCST = 1.3ln(Mn -5.4). S11 LCSTheating (oC) Figure S12: Plot demonstrating the effect of HEAm loading on the LCST of poly(NIPAm-r-HEAm) copolymers and the hysteresis observed between LCSTheat ing and LCSTcooling . The legend refers to the relative loadings in mol % of NIPAm:HEAm in the copolymer. HEAm Composition (%) Figure S13: Plot demonstrating the change in LCSTheat ing of poly(NIPAm-r-HEAm) copolymers as a function of copolymer composition. S12 S.3 DOX Release from Poly(N-Isopropylacrylamide) Particles Preparation of Doxorubicin (DOX) loaded Poly(NIPAm) particles. An aqueous solution of PNIPAm (10% w/v) and DOX (0.5% w/v) was kept at 4 ◦ C for 12 hours to allow the polymer to dissolve. The polymer/drug solution was then added dropwise into 50 mL of silicon oil kept at 40 ◦ C. The polymeric particles were washed with hexane 5 times and air dried overnight. DOX release (%) Release study of DOX-loaded particles. The release profile of DOX from DOX-loaded particles was determined by dialysis. Poly(NIPAm)-DOX particles (10 mg) were dissolved in water to form a 1 mg/mL Poly(NIPAm)-DOX particle solution containing 45 µ g DOX. This solution was loaded into MWCO 100 kDa dialysis tubing and dialyzed against 7 mL of phosphate buffered saline (PBS, pH 7.4) in the dark at 37 ◦ C. At specified time points, 1 mL of the dialysis buffer was collected and replaced with equal volume of fresh PBS. The concentrations of DOX present in the dialysate were determined by measuring absorbance at 480 nm. The concentration of DOX released from the micelles was expressed as a percentage of the total DOX concentration and plotted as a function of time. In order to study the effect of temperature on the kinetics of release, a similar experiment was carried out but with the temperature maintained at 4 ◦ C. DP 100, k = 5.57 % min-1 DP 1000, k = 3.16 % min-1 DP 5000, k = 2.32 % min-1 Time (min) Figure S14: Plot of the release of DOX from poly(NIPAm) particles. S13 Figure S15: Plot demonstrating the effect of poly(NIPAm) molecular weight on the observed release rate constant of DOX from polymer particles in aqueous buffer at 37 o C. The plot demonstrates a logarithmic relationship between the Mn in kDa and the release kinetics in %/min according to the following equation: Release Rate = -0.85ln(Mn )+9.33. S14