Introduction:
advertisement

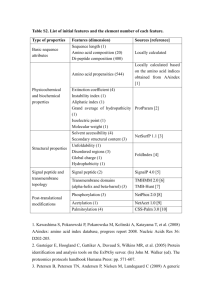
Introduction: Olfaction is a major neurosensory function by which mammals learn about the external environment. The initial step involved in olfaction is the interaction of the odorant with the olfactory receptors (ORs). To recognize odors corresponding to different odorants, a combinatorial approach has been developed in mammals. There are 100’s of different ORs in each mammal. Each odorant binds to multiple ORs and each OR binds to multiple odorants to generate different activation patterns for different odorants (Malnic, et. al., 1999, Firestein, 2001). ORs make up the largest family of genes in the human genome and belong to a superfamily of GTP-protein binding coupled receptors (GPCRs) (Mombaerts, 1999). Most enzyme families have developed specificity towards certain ligands and hence the binding sites in these families display a high degree of conservation. However, as the ORs have to recognize numerous diverse odorants, a large repertoire of proteins has been developed with binding site sequence variability (Pilpel & Lancet, 1999). Such variability in the sequence profiles is also known to occur in immunoglobulins, T cell receptors and major histocompatibility complex proteins. It has also been hypothesized that the odorant binding sites are present in transmembrane segments 3, 4 and 5 where the highest sequence variability is found. It has been postulated that since most good ligands for metal ions form better odorants (methylthiol binds to some OR > 1 million times stronger than the OR that corresponds to methanol), they must be coordinated to a metal ion bound in an OR (Wang, et. al., 2003). Knowledge about the three dimensional structural structure of ORs is crucial for understanding how they function and also for drug design. However, as membrane proteins are difficult to crystallize, it has been difficult to gain any structural information about the ORs yet. GPCRs are membrane bound proteins that consist of seven transmembrane helices. Here, we have used the structure of bovine rhodopsin, a GPCR, and other homologous proteins such as bacteriorhodopsin, sensory rhodopsin and halorhodopsin to generate models of the structure for a olfactory receptor, O2D2. Previous reports in the literature have used just one of the above structures to generate models for the olfactory receptor but the presence of other templates could improve the model (Floriano, et. al., 2000, Vaidehl, et. al., 2002). Modeling Methods: Prediction of Transmembrane helices: Prediction of transmembrane helices of the olfactory receptor O2D2 was done using TMHMM v. 2.0 (Sonnhammer, et. al., 1998). It was predicted to be a seven helix bundle similar to GPCR as shown in Fig. 1A. It is interesting to note that the putative metal binding domain is predicted to be in the loop between helices 4 and 5. This is because of the presence of the charged residue E180 in O2D2. However, upon metal binding, this region becomes neutral. On creating a mutation E180V corresponding to the metal bound neutral residue, there are 8 putative transmembrane helices (Wang, et. al., 2003) as seen in Figure 1b. As the templates used in this study have 7 transmembrane helices, it is expected that the model will also have 7 transmembrane helices. The above analysis indicated that the olfactory receptor might form an eight-helix bundle after metal binding. Alignment of O2D2 to rhodopsin: The templates used for modeling were 1L9H (bovine rhodopsin) (Okada, et. al., 2002), 1E12 (halorhodopsin) (Kolbe, et. al., 2000) and 1JGJ (sensory rhodopsin) (Luecke, et. al., 2001). It has been suspected for the GPCRs, there will not be much difference in the placement and angle of the transmembrane helices and hence these are good templates for our modeling. These templates were chosen on the basis of best resolution and least residues missing in the structure. The structure of bacteriorhodopsin was not used in this study because there were 20 residues missing in its high-resolution structures. The structural alignment, in which all the structurally similar regions are aligned, of these three structures was done using STAMP v.4.2 (Russel & Barton, 1992). Structural alignment based methods are better than the sequence alignment based methods. In Figure 2, we can see that the transmembrane helices of the three structures align well though the helices in bovine rhodopsin are larger than those in both the archaea rhodopsins. Based on the alignment from STAMP, a profile for this family was created using HMMER v.2.1 (Eddy, 1998). HMMER creates a hidden Markov model of the sequence and can be used to search sequences from a database that belong Figure 1A Figure 1B Figure 1: Secondary structure prediction of the human olfactory receptor O2D2 using TMHMM v. 2 for A. the native sequence and B. the mutant E180V. On the top, the thick red bars indicate predicted transmembrane helices, the thin blue lines indicate cytoplasmic region and the think pink line indicates extracellular region. Figure 2: top: The primary sequence and secondary structure alignment of structural alignment rhodopsin family. Here, cylinders indicate helical regions in that structure and arrows indicate beta sheet region. Bottom: The alignment of the structures in ribbon representation. Here green represents 1JGJ, brown represents 1E12 and white represents 1L9H. Figure was developed using VMD (Humphrey, et. al., 1996). to a particular family. To check how good this profile was, we searched for proteins belonging to this class using this profile in the SWISSPROT database (Boeckmann, et. al., 2003). The profile recognized more than 400 proteins (data Figure 3: The profile of the O2D2 olfactory receptor family. not shown) from the SWISSPROT database, which belonged to the GPCR superfamily and had no false positives, which shows the profile was able to differentiate between proteins belonging to the GPCR superfamily from other superfamilies. It has been reported that it is better to align a profile of the sequence to the structural profile for GPCRs because the regions that are conserved over the sequence profile give a stronger signal than the individual sequence. The profile for O2D2 was made using CLUSTAL W from homologous non-redundant sequence in the SWISSPROT database. In figure 3, we have the sequence profile of O2D2. Figure 4: The alignment in the profile of O2D2 family with the structural profile of the rhodpsin family. Before we carry out the modeling of O2D2, we have to make sure that the alignment we get is correct. There are some conserved sequence motifs that help to identify the position of the transmembrane segments in the GPCR subfamilies (Mirzadegan, et. al., 2003). It is seen that the O2D2 subfamily also has these elements (Pilpel, 1999, Zozulya, et. al., 2001, Fuchs, et. al., 2001) and hence these motifs can be used to align the transmembrane segments in O2D2 Figure 5: A: Ribbon representation of the model structure of the olfactory receptor O2D2. B: The space filling model of the model with polar residues shown in green, positively charged residues in blue and negatively charged residues in red. to those in the rhodopsin structures. We have used HMMER to align the O2D2 profile to the structural profile created from the rhodopsin structures. In helix 1 of GPCR families, the highly conserved G20 and N21 (numbering starts form beginning of helix) are also conserved in the O2D2 family – G41 and N42 (the position refers to the alignment position of the family). Similarly in helix 2, D70 of O2D2 aligns with the highly conserved D13 of helix 2 in the GPCR superfamily. For helix 3, the DRY motif starting at 121 is highly conserved starting from 28 th position of the 3rd helix. Also to be noted that C97 is conserved in all these proteins because it is suspected that C97 forms a disulfide bond with C179 present in the previously reported extracellular loop. In helix 4, the fully conserved W158 of O2D2 was aligned to 11th position of the 4th helix. HMMER aligned the first four helices satisfying the above criteria. HMMER aligned the earlier reported extracellular loop between TM4 and TM5 to TM5 of the rhodopsin family. However, as already stated above, it has been suspected that O2D2 could be an eight-helix bundle when the HFFCE motif is bound to metal ion forming the 5th helix. This would mean that the previously reported TM5, TM6 and TM7 of O2D2 now form the 6th, 7th and 8th helices respectively. On analysis of the alignment created by HMMer in Figure 4, it can be seen that this is indeed the case. However, as the rhodopsin family has only 7 helices, there is no corresponding structural element for the 8th transmembrane helix to align to with the rhodopsin family as shown in Figure 4. Modeling of O2D2: Modeling of O2D2 with the 3 templates was carried out using MODELLER v.6.2 (Sali & Blundell, 1993) using restraints on the secondary structural elements and also high refine level for ab initio structure prediction of loop regions. The model of O2D2 that we obtained is shown in Figure 5A. The space-filling model of the structure is shown in Figure 5B. It is analyzed to check whether the residues are in the proper environment. In particular we check whether any charged residues are exposed to the lipid. On similar analysis with the rhodopsin family, it was observed that no charged residues are exposed to the lipid bilayer towards the central region of the helix. Similarly, none of the charged residues are exposed to the lipid in the middle of the membrane in the model of O2D2. Conclusions: A 3-dimensional model of the structure of O2D2 was developed with three templates. The loops and the C terminal region have to be refined further. It has been believed that the olfactory receptors have 7 transmembrane helices. However, our work suggests that O2D2 might have eight membrane spanning domains. However, it could be that O2D2 starts of as a seven transmembrane helix and then on binding the metal ion, the EC2 loop (2 nd extracellular loop) enters in to the membrane in the place of TM4. It has already been stated based on the variability profiles of sequence in different regions of the protein that EC2 and TM3-5 are involved in odorant recognition and binding and this model could be used for those studies (Pilpel & Lancet, 1999). Hence the binding region of the olfactory receptors is very similar to that of bacteriorhodopsin which also uses TM3-5 and EC2 for retinol binding. It has also been shown that the amino acids involved in odorant binding for O2D2 are the ones which point towards each other as shown in Figure 6. Future work would involve trying to model O2D2 with HFFCE in EC2 and also in TM4 and TM5 positions with TM4 and TM5 as the loops on the intracellular side. After this, a steered molecular dynamics study could help in identifying how tightly the protein binds to the membrane and this will help in knowing the stable conformation of O2D2 and hence some studies on the function of the protein can be done after this. Figure 6: 2-dimensional representation of the OR seven TM segments. Conserved residues are shown. In addition, OR positions that align with ligand contact residues in other GPCRs are colored green; OR variable positions that donot align with such residues are colored red. Circles are OR variable positions, while squares are OR conserved positions.The conserved regions are shown in squares and the variable regions in green. Experimental work could involve knowing the exact role of EC2 in the functioning of the protein and to see whether metal ions bind to the olfactory receptor both in proteins such as wildtype O2D2 and mutant O2D2 with the mutation E180V. Further, the exact position of the transmembrane helices could help in improving the model. This can be done by labeling studies. References: Boeckmann B, Bairoch A, Apweiler R, Blatter MC, Estreicher A, Gasteiger ME , Martin MJ, Michoud K, O’Donovan C, Phan I, Pilbout S, Schneider M. The SwissProt protein knowledgebase and its supplement TrEMBL in 2003. Nucleic Acids Residues 2003, 31:365-370. Eddy, SR: HMMER: Profile hidden Markov models for biological sequence analysis. (http://hmmer.wustl.edu/) Firestein, S: How the olfactory system makes sense of scents. Nature 2001, 413:211-218. Floriano WB, Vaidehl N, Goddard WA, Singer MS. Shepherd GM: Molecular mechanisms underlying differential odor responses of a mouse olfactory receptor. Proceedings of National Academy of Sciences, USA 2000, 97:10712-10716. Fuchs T, Glusman G, Saban SH, Lancet D, Pilpel Y: The human olfactory subgenome: from sequence to structure and evolution. Human Genetics 2001, 108:1-13. Humphrey W, Dalke A and Schulten K: “VMD- Visual Molecular Dynamics.” Journal of Molecular Graphics 1996, 14:33-38. Kolbe M, Bessir H, Essen LO, Oesterhelt, D: Structure of Light-Driven Chloride Pump Halorhodops at 1.8 A Resolution. Science 2000, 288: 1390-1396. Luecke H,Schobert B, Lanyi JK, Spudich EN Spudich JL: Crystal Structure of Sensory Rhodopsin II at 2.4 Angstroms: Insights Into Color Tuning and Transducer Interaction Science 2001, 293: 1499-1503. Malnic B, Hirono J, Sato T, Buck LB: Combinatorial receptor codes for odors. Cell 1999, 96:713-723. Mirzadegan T, Benko G, Filipek S, Palzzewski Z: Sequence Analysis of GProtein Coupled Receptors: Similarity to rhodopsin. Biochemistry 2003, 42:27592767. Mombaerts P: Seven-transmembrane proteins as odorant and chemosensory receptors. Science 1999, 286:707-711. Okada T, Fujiyoshi Y, SilowM, Navarro M, Landau EM, Shichida Y: Functional Role of Internal Water Molecules in Rhodopsin Revealed by X-Ray Crystallography Proeedings of National Academy of Sciences USA 2002, 99: 5982-5987. Pilpel Y and Lancet D: The variable and conserved interfaces of modeled olfactory receptor proteins. Protein Science 1999, 8:969-977. Russell RB and Barton GJ: Multiple protein sequence alignment from tertiary structure comparison. Proteins Structure, Function and Genetics 1992, 14:309323. Sali A and Blundell TL: Comparative protein modelling by satisfaction of spatial restraints. Journal of Molecular Biology 1993, 234: 779-815. Sonnhammer ELL, von Heijne G, and Krogh A: A hidden Markov model for predicting transmembrane helices in protein sequences. Proceedings of the Sixth International Conference on Intelligent Systems for Molecular Biology 1998, 175182. Vaidehl N, Floriano WB, Trabanino R, Hall SE, Freddolino P, Choi EJ, Zamanakos G, Goddard WA: Prediction of structure and function of G Proteincoupled receptors. Proceedings of National Academy of Sciences, USA 2002, 99:12622-12627. Wang J, Schulten ZL, Suslick, KS: Is the olfactory receptor a metalloprotein? Proceedings of National Academy of Sciences, USA 2003, 100:3035-3039. Zozulya S, Echeverri F, Nguyen T: The human olfactory repertoire. Genome Biology 2001, 2:0018.1-0018.12