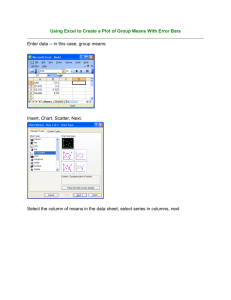
What compound is being analyzed for in in the chocolate
advertisement

Name __________________________ (15) SCHA261 Exam 1 (100) Pass in Your Formula/Notes Sheet with your exam (make sure your name is on it) 1) What compound is being analyzed for in the chocolate bar example in Chapter 0 of the text? (5) Caffeine 2) A sheet of cardboard has the following dimensions. A length of 279.0 mm by a width of 216.0 mm and the sheet is 5.2 mm thick. What is the volume of this piece of cardboard? V = lwh a) Express the answer using the significant figure rules. (5) 3.1 x 105 mm3 b) Express the answer using propagation of error. (this will give you a + format for your answer) (5) re = 0.019 3) abs error is 6028 therefore 31300 + 6000 mm3 How many Liters are in 19.48 m3 ? (10) 1.948 x 104 L 4) A quality control scientist is carrying out analysis for the amount of aspirin in a tablet. The following results are obtained: 81.2 mg, 82.4 mg, 81.6 mg and 81.9 mg. The specification for this type of table is 81.0 mg. Are these results consistent the specification amount at the 95% confidence level? (15) Ave 81.77 so 81.8 mg consistent s = 0.5058 so 0.5 mg t calc = 1.60 ttab = 3.18 so results 5) Another group in the lab carries out additional analyses on this lot of tablets. Their results are reported to you as 82.4 ± 0.6 mg. They did 5 replicate analyses. Are the two sets of analyses consistent at the 90% Confidence? Use the 0.7 mg as your pooled standard deviation. You need not calculate this pooled value for this problem. (15) T calc = 1.27 results are same 6) Three groups are working on the same assay using the same method. An F test has been carried out and it has shown that are the standard deviations are not significantly different. The following results were obtained: Group A: 81.7 ± 0.5 mg, Group B: 82.0 ± 0.3 mg and Group C: 80.8 ± 0.6 mg. What is the pooled standard deviation of this data if each group did four determinations? (10) S pooled = 0.483 mg sig figs are off for this but I want you to see the value. 7) This group determined the following for the amount of aspirin in another formulation of tablets. 254.3 mg ± 7.8 mg. Express this experimental error as a relative error in terms of parts per thousand. (10) Re = 31 ppt 8) What is the Molarity of Concentrated Hydrofluoric acid? This very hazardous acid has a weight percent of 49.0 g/g, a density of 1.30 g/mL and has a formula weight of 20.01 g/mol. (10) 31.8 M 9) What is meant by precision in an analytical result? (5) Ability to repeat a result. Table I. Values of Student’s t 50 Confidence Level (%) 90 95 98 99 99.9 6.313752 12.7062 31.82052 63.65674 636.6192 2.919986 4.30265 6.96456 9.92484 31.5991 2.353363 3.18245 4.5407 5.84091 12.924 2.131847 2.77645 3.74695 4.60409 8.6103 2.015048 2.57058 3.36493 4.03214 6.8688 Degrees Freedom 1 2 3 4 5 1 0.816497 0.764892 0.740697 0.726687 6 7 8 9 10 0.717558 0.711142 0.706387 0.702722 0.699812 1.94318 1.894579 1.859548 1.833113 1.812461 2.44691 2.36462 2.306 2.26216 2.22814 3.14267 2.99795 2.89646 2.82144 2.76377 3.70743 3.49948 3.35539 3.24984 3.16927 5.9588 5.4079 5.0413 4.7809 4.5869 11 12 13 14 15 0.697445 1.795885 0.695483 1.782288 0.693829 1.770933 0.692417 1.76131 0.691197 1.75305 2.20099 2.17881 2.16037 2.14479 2.13145 2.71808 2.681 2.65031 2.62449 2.60248 3.10581 3.05454 3.01228 2.97684 2.94671 4.437 4.3178 4.2208 4.1405 4.0728