Protection from Blood Transfusion Induced Sensitization by
advertisement

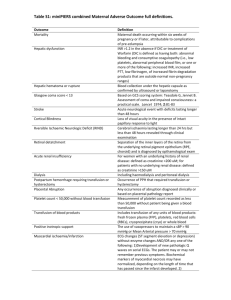
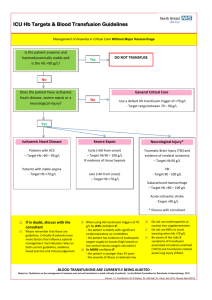
Renal: Protection of Blood Transfusion Induced Sensitization by Cyclosporin A Doc# Guideline and Procedure Protection from Blood Transfusion Induced Sensitization by Cyclosporin A Document Number: Sites where Guideline and Procedure applies: Target audience: All HNE facilities where a patient undergoes haemodialysis Nephrology clinical staff, who provide care to haemodialysis patients. This document comprises part of the clinical information package for care of Haemodialysis patients. Haemodialysis, Description: Keywords: Replaces Existing Guideline and Procedure: Yes Registration Number(s) and/or name and JHH Nephrology SWP N.1.6 of Superseded Documents: Relevant or related Documents, Australian Standards, Guidelines etc: NSW Health Policy Directive 2007_079 Correct patient, Correct procedure, correct site http://www.health.nsw.gov.au/policies/pd/2007/pdf/PD2007_079.pdf NSW Health Policy PD 2005_406 Consent to Medical Treatment http://www.health.nsw.gov.au/policies/PD/2005/pdf/PD2005_406.pdf NSW Health Policy Directive PD 2007_036 Infection Control Policy http://www.health.nsw.gov.au/policies/pd/2007/pdf/PD2007_036.pdf NSW Health 2007_007 Medication Handling in NSW Public Hospitals. http://www.health.nsw.gov.au/policies/pd/2007/PD2007_077.html John Hunter Hospital Nephrology Pharmacist Product Information Leaflet Prerequisites (if required): Registered or Endorsed Enrolled Nurses Valid medication order Procedure Summary: This Guideline and Procedure sets out the steps to be followed when administering Cyclosporin A to patients who are on the Transplant List and require a blood transfusion. The procedural components of the document such as, Preparation of patient, Preparation of equipment, Technique, Cleaning up and Documentation are considered mandatory. Guideline Note : This document reflects what is currently regarded as safe and appropriate practice. However in any clinical situation there may be many factors that cannot be covered by a single document and therefore does not replace the need for the application of clinical judgment in respect to each individual patient. Date authorised: Authorised by: HNEAHS Renal Clinical Stream Leadership Group Contact Person: Contact Details: Review due date: TRIM Number: Version No. 1 December 2010 Page 1 of 3 Renal: Protection of Blood Transfusion Induced Sensitization by Cyclosporin A Doc# OUTCOMES 1 Safe administration of Cyclosporin A to a patient who is on the transplant List 2 Avoid Blood Transfusion exposure to foreign HLA antigens ABBREVIATIONS & GLOSSARY mg Milligram kg Kilogram bd Twice daily CMV Cytomegalovirus HLA Human leukocyte antigen GUIDELINE Blood Transfusion exposes the patient to foreign HLA antigens that may result in the development of lymphocytotoxic antibodies that can interfere with transplantation by increasing the difficulty of the tissue matching and increasing the risk of acute rejection. All patients on the transplant list or those who are likely to be on the Transplant list at some time should receive Cyclosporin A to protect from sensitization; the regime is a dose the day before, the day of, and the day post transfusion . This is called peri-transfusion Cyclosporin. As dialysis patients are more likely to require a blood transfusion at some point during their dialysis treatment lifetime, it is vital that dialysis nursing staff remain up to date on their patients transplant status. If a patient requires an urgent blood transfusion, the Transplant Recipient Coordinator may be contacted after hours for confirmation of the patient’s listing on the transplant waiting list or undergoing assessment for the same (page 5266 through John Hunter Hospital switch board). Dosing Peri-transfusion Cyclosporin Dosing Schedule: Cyclosporin 4mg/Kg bd (rounded to the nearest 25mg) on day minus 1, day 0 and day 1, with respect to every transfusion. Gastric irritation is dose related; hence the dose 8mg/kg/day (rounded to the nearest 25mg) is divided into two. Note: When transfusion is urgent, as much as possible of this protocol should be achieved, but there is no point in extending the treatment past day 1 after transfusion. Note: If additional transfusions are indicated medically the additional Cyclosporin A will be given. Note: All CMV negative recipients should receive CMV negative blood and this should be specified on the pathology cross match for the blood product. In many rural areas staff will need to alert blood bank to allow sufficient time to transport the CMV negative blood to their location. APPENDICES Five Moments for Hand Hygiene REFERENCES Daugirdas, Blake & Ing. Handbook of Dialysis 3rd ed. Philadelphia, Lippincott Williams & Wilkins E-MIMS Mathew J Cervelli, February 2007, The Renal Drug Reference Guide (first edition) NSW Health PD 2007-077: Medication Handling in NSW Public Hospitals Version No. 1 December 2010 Page 2 of 3 Renal: Protection of Blood Transfusion Induced Sensitization by Cyclosporin A Doc# Appendix 1 Adopted from the World Health Organization and Hand Hygiene Australia. Version No. 1 December 2010 Page 3 of 3