Effect of Different pH Buffers and Different
advertisement

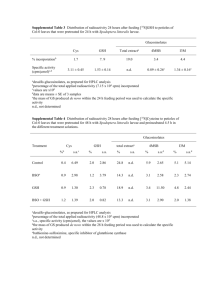
EFFECT OF DIFFERENT PH BUFFERS AND DIFFERENT TEMPERATURES ON LITHIUM CARBONATE-GLUTATHIONE INTERACTION Hashmat Ullah1, Muhammad Farid Khan1 & Farwa Hashmat 2 1 Department of pharmaceutical Chemistry, Faculty of Pharmacy Gomal University, Dera Ismail Khan 1 Department of Chemistry, Gomal University, Dera Ismail Khan ABSTRACT Physiological pH and body temperature are both very important in the life of an organism because different bio-chemical reactions take place at specific pH as well as on specific temperature. The rate of a particular bio-chemical reaction may increase or decrease due to slight alteration in either pH or temperature or both. Keeping in view this fact, we have studied the affect of lithium carbonate on GSH chemical status (reduced glutathione) at six different pH and at three different temperatures by using Ellman’s modified method. Our results show that lithium carbonate shows better interaction at pH 7.5 and temperature 35oC which are both near to physiological pH and body temperature i-e lithium carbonate cause decrease in GSH concentrations near the physiological pH and near body temperature which may be due to the formation of Li-S-G complex or GSSG. Keywords: Physiological pH, Temperature, Lithium Carbonate (Li2CO3), GSH (Reduced Glutathione) INTRODUCTION many reactive metabolites by spontaneous During the process of evolution, O2 conjugation or by a reaction which is tension increases in the environment as a catalyzed by the GSH S-transferases is the result aerobic organisms need a strong main role of reduced form of glutathione. system for restoring sulfhydryl group to (Coles et al., 1990; Hinchman reduced form after exposure to oxidation 1994). Reduced form of glutathione has stress.(Fridovish, 1989; Naqui et al., strong affinity for metals and it acts as 1986), without a process to reduce protein metal binding ligands as this form of disulfides, cysteinyl residues of essential glutathione has very important role in enzymes might remain oxidized, leading transport, storage and metabolism of to changes in catalytic activity, this metals. Glutathione is found in millimolar function is full filled by the thiol-disulfide concentrations in exchange catalyzed by thiol-transferases in 2000), brain is very sensitive to oxidative the presence of GSH (Sohal et al., 1996; damage than rest of the body tissues Sundquist et al., 1989). Detoxification of (Halliwell et al., Gomal University Journal of Research, 28(1). June, 2012 et al., brain (Dringen et al., 1986) while GSH 2 Hashmat et al., Effect of Different pH depletion weakens the defense system of behavioral responses body and hence cause progression of chlinergically mediated. Thus, lithium disease. Administration of reduced GSH reduces the convulsant threshold to the showed improvement in patient with nonspecific cholinergic muscarinic agonist PD.(Sechi et al., 1996). are coline and to anticholinesterases (Jope, Serotonergic neurotransmission increases 2001). when lithium is used for short term (Price, decreases sensitivity of CNR (Central et al., 1990; Shiah, et al., 2000) but this nicotinic receptors) (Want et al., 1997). Lithium that are administration also effect is not observed after long-term treatment and therefore the implications of MATERIALS AND METHODS this in the mechanism of action of lithium in long term treatment and in its anti- MATERIALS suicide effect are unclear (Lenox, et al., Reduced 1995).Dopaminergic system can also be Lithium effected Sodium by lithium. neurotransmission is Dopamine shown to be glutathione carbonate (GSH) (BDH, hydroxide, (Fluka), Germany), DTNB Dithiobis-2-Nitrobenzoic acid i-e5,5(Sigma), decreased during acute administration of KH2PO4 (Merck), Double refined distilled lithium which may be link to lithium’s anti water (D/W), HCl 35% (Kolchlight), manic effect. Consistent turnover of Oven: Memmert Model U-30 854 (Schwa chronic lithium is Boch, Germany), UV-Spectrophotometer Behavioral & (UV-1601 Shimadzu, Japan), Magnatic HIDRS stirrer (Hungary), Micropippete 200 µl, (Haloperidol induced dopamine receptor 500 µl, 1000 µl (Finnpipette Digital supersensitivity) can not be prevented by Finland), chronic lithium treatment. (Lenox et al., Electrical balance type AX200 (Shimadzu 2000).Chronic administration Corporation, Japan), pH Meter: model produces an up regulation of muscarinic Nov-210 (Nova scientific Company Ltd, receptors in the rat brain (De Bruin et al., Korea). All chemicals used in this research 2000). Studies have also shown that were high quality chemicals and were used lithium treatment enhances various as purchased without further purification. however administration very biochemical little. symptoms lithium of of Disposable Gomal University Journal of Research, 28(1). June, 2012 rubber gloves, 3 Hashmat et al., Effect of Different pH METHODS 26.66 µM, 40.00 µM, 53.33 µM, and Preparation of Stock Solutions: 66.66 µM respectively starting from lower 15.4mg of GSH (reduced glutathione) was used concentrations of GSH to higher used added in 50ml of 0.2M buffer (phosphate) concentration of GSH. From each GSH pH7.6 to prepare 1mM GSH solution dilution 0.2ml was mixed with 2.3 ml of while a few drops of 0.1 N HCl were also buffer (phosphate) having pH 7.6 then added to it and the prepared solution was 500µl DNTB was mixed to this and these kept in refrigerator till further use. 1ml of mixtures were incubated for five minutes. 1mM GSH stock solution was taken and After five min:, ABS( absorbance )of each diluted with 9ml phosphate buffer pH 7.6 sample was recorded at fixed wavelength to prepare 10ml of 0.1mM GSH solution. λ max: 412 nm. 1mM DTNB solution was prepared by DTNB Blank dissolving 19.8mg of DTNB in 50ml Solution of DTNB blank was prepared by phosphate buffer pH 7.6. 1mM Li2O3 mixing 500µl of DTNB solution (1mM solution was prepared by dissolving DTNB) with 2500 µl of buffer (phosphate) .369mg in 50ml distil water. To prepare pH 7.6 and ABS ( absorbance) of DTNB 0.1mM lithium carbonate (Li2O3) solution, blank solution was also recorded at λ max: 1.0ml of 1mM Li2CO3 stock solution was 412 nm. taken into a test tube and was diluted with Real Absorbance distil water up to 10ml. By subtracting absorbance of DTNB blank Standard Curve from the absorbance of each sample Standard curve was drawn by preparing (containing four dilutions from 1mM GSH (Reduced absorbance of each sample was obtained. form of Glutathione) while 5th dilution was All the obtained ABS were converted into 1.0mM GSH solution itself. The dilutions GSH concentration by using equation were in sequence of 200µM, 400µM, given below and a standard curve was 600µM, 1000µM. obtained reduced Equation 800µM and Concentration (Final) of glutathione in dilutions was 13.33µM, GSH + y = mx + b Gomal University Journal of Research, 28(1). June, 2012 DTNB), real 4 Hashmat et al., Effect of Different pH Fig 1: Standard Curve for Glutathione (GSH) Affect of pH6.5, pH7.5, pH8.5, pH9.0, recorded against reference cell containing pH9.5, pH10.0 Buffer Solutions on 2.8ml buffer and 0.2ml of 0.1mM GSH at Lithium fixed wavelength λ max 412nm. As each Carbonate-Glutathione Interaction time sample mixture was in different pH pH6.5, pH7.5, pH8.5, pH 9.0, pH 9.5, buffer so reference cell also contain buffer pH10.0 buffer solutions were prepared and of same pH as in sample cell. Control 2.3ml from each buffer was taken and solution separately mixed with 2ml of 0.1mM glutathione was also prepared by taking glutathione solution in six test tubes, then 4.3ml of each buffer solution in six test 1.0ml 5,5-dithiobis-2- tubes separately. 2.0ml of 0.1mM solution nitrobenzoic acid i-e DTNB was added to of glutathione was added to test tube this, all these six mixture were well shaken following by the addition of 1.0ml of for 10 minutes and to each of the six 1.0mM DTNB. After 5 minutes 3.0ml mixtures lithium from each test tube was taken in cuvettes. carbonate (Li2CO3) was added. These GSH final concentration in control sample mixtures were again very well shaken and was 27.4µM (0.027mM) as in the sample. incubated for 5 minutes. After 5 minutes, ABS( Absorbances) were recorded after 3ml from each mixture was taken into five minutes at fixed wavelength λ max sample curette turn by turn and ABS was 412nm against reference cell containing of 1mM 2.0ml of 0.1mM (glutathione Gomal University Journal of Research, 28(1). June, 2012 blank) for 5 Hashmat et al., Effect of Different pH 2.8 ml of respective phosphate buffers and ABS( absorbances) which were then 0.2 ml of 0.1mM glutathione. Effect of converted into conce: of glutathione Li2CO3 on the chemical status of GSH (Table 1) in mixture by well known (reduced glutathione) in pH6.5, pH7.5, modified Ellman’s method as described pH8.5, pH9.0, pH9.5, pH10.0 buffer in standard curve for GSH( reduced solutions was studied by determining the glutathione). Table #1: Effect of pH6.5, pH7.5,pH8.5,pH9.0,pH9.5,pH10.0) Buffer Solutions on chemical Status of Glutathione (GSH) with and without the presence of Lithium Carbonate ABS i-e absorbance of 5,5-Dithiobis-2-Nitrobenzoic acid (DTNB) Blank solution was 0.057 ABS at fixed wavelength 412nm Final Mixture contained GSH concentration 27.3µM Real abs*/conc. of GSH after reaction ABS(Real)*/conc. Mean of 3 with Lithium of GSH Blank Readings Carbonate Lithium S# pH Carbonate ABS ABS final conce: st nd (1 ) (2 ) ABS rd (3 ) in Mixture Abs. Conc. of GSH(µM) Abs. Conc. of GSH(µM) 1 6.5 27.3 µM 0.481 0.478 0.475 0.478 0.421 2.72 0.761 4.89 2 7.5 27.3 µM 0.444 0.443 0.440 0.442 0.385 2.49 0.745 4.78 3 8.5 27.3 µM 0.454 0.452 0.447 0.451 0.394 2.55 0.753 4.83 4 9.0 27.3 µM 0.494 0.488 0.485 0.489 0.432 2.79 0.767 4.92 5 9.5 27.3 µM 0.512 0.509 0.507 0.509 0.452 2.92 0.772 4.96 6 10 27.3 µM 0.532 0.529 0.526 0.529 0.472 3.04 0.785 5.04 * ABS(Real)= ABS of Mixture - ABS of DTNB blank Solution. Gomal University Journal of Research, 28(1). June, 2012 6 Hashmat et al., Effect of Different pH Figure 2: Effect of different pH buffers on Lithium Carbonate-GSH interaction in aqueous medium Effect of different pH buffers on lithium carbonate GSH interaction. GSH control. Results are the mean ±SE of 3 experiments. Affect of Different Temperatures i-e 25 0.5 ml of 1mM DTNB stock solution was Degree Celsius, 35 Degree Celsius, 45 added into each test tubes (sample Degree Celsius) on Lithium Carbonate- mixture) GSH Interaction glutathione and lithium carbonate in the 2.0ml of concentration of sample test tube was 0.0033mM (3.33µM) solution was added separately to 2ml of respectively. Control solution (glutathione 0.1mM glutathione taken in three separate blank) for glutathione was also prepared test (reaction by taking 2ml of 0.1mM glutathione stock mixtures) were well shaken and were kept solution in a test tube to which 2ml of in water bath for 10 minutes to maintain phosphate buffer having pH 7.6 was the temperature of (25 degree Celsius, added. 35oC, 45 degree Celsius). Glutathione and glutathione in control solution was also lithium carbonate final concentration in 0.05mM (50µM) as in reaction mixture. each of the reaction mixture was 0.5mM 0.2ml was taken from this sample and (50µM) respectively. 2300µl (2.3 ml) of phosphate buffer Sample cuvettes were prepared by taking solution was mixed/added to it following 0.2ml of Li2CO3 plus glutathione mixture by addition of 500µl of 1mM DTNB. The from each one of the previously made test ultimate final concentration of glutathione tubes (reaction mixture) and diluted with in 2.3ml of phosphate buffer pH 7.6 and then (3.33µM). ABS (absorbance) was taken These lithium final carbonate tubes. 0.1mM the mixtures The control final sample Gomal University Journal of Research, 28(1). June, 2012 concentration was of 0.0033mM 7 Hashmat et al., Effect of Different pH after five minutes at λ max 412nm against temperature was studied in terms of reference cell containing 2.8ml buffer determination of the absorbances which solution (phosphate) having pH 7.6 and were then converted into concentration of 0.2ml of 0.1ml glutathione solution. The glutathione (Table2) in mixtures by a well affect of lithium carbonate(Li2CO3)on the known Ellman’s method, as mentioned in chemical status of glutathione at different standard curve for glutathione. Table #2: Effect of temperatures i-e 25 degree Celsius,35 degree Celsius,45 degree Celsius on chemical Status of Glutathione (GSH) 0.1mM ABS of 5,5-dithiobis-2-nitrobenzoic acid (DTNB) blank solution was 0.060ABS at fixed wavelength 412nm In final Mixture GSH concentration is 3.33µM Real abs*/conc. of Final Conc. S# GSH after reaction ABS(Real) */conc. of GSH Blank Temper Of Lithium ABS ABS ABS Mean of 3 with Lithium ature Carbonate 1st 2nd 3rd Readings Carbonate in Mixture Abs. Conc. of GSH(µM) Abs. Conc. of GSH(µM) 1 25 oC 3.33 µM 0.299 0.297 0.295 0.297 0.237 1.55 0.441 2.85 2 35 oC 3.33 µM 0.278 0.276 0.275 0.276 0.216 1.41 0.432 2.79 3 45 oC 3.33 µM 0.311 0.306 0.301 0.306 0.246 1.61 0.427 2.69 *ABS (Real) = ABS of Mixture - ABS of DTNB blank Solution. Gomal University Journal of Research, 28(1). June, 2012 8 Hashmat et al., Effect of Different pH Figure 3: Effect of different Temprature (25 degree Celsius, 35 degree Celsius, 45 degree Celsius) on lithium carbonate-glutathione interaction in aqueous medium Effect of different temperature on lithium carbonate GSH interaction. GSH control. Results are the mean ±SE of 3 experiments. RESULTS the remaining concentration of GSH was Affect of pH6.5, pH7.5, pH8.5, pH9.0, left minimum (Table 1 and Fig1) in buffer pH9.5, pH10.0 Buffer Solutions on pH 7.5 indicating that this buffer with pH Li2CO3-GSH Interaction in Aqueous 7.5 is favorable for interaction between Medium. Li2CO3 and GSH (near to body pH). Our To study the affect of lithium carbonate results show that although Li2CO3 reacts (Li2CO3) on the chemical status of GSH with GSH in different pH buffer but it (reduced form of glutathione) in different cause pH buffers, we have prepared buffers of concentrations in buffer pH 7.5 indicating six different pH i.e. pH 6.5, pH 7.5, pH7.8, that lithium in the same way might be the pH 9.0, pH 9.5 and pH 10.0 and it was cause of GSH depletion in human body. found that Li2CO3 has best interaction in The decrease of GSH in buffer pH 7.5 buffer pH 7.5 which is very close to after the reaction with lithium carbonate physiological pH of human body. After the can reaction of lithium carbonate ( Li2CO3 ) concentration in the same buffer of pH 7.5. greater be depletion compared Gomal University Journal of Research, 28(1). June, 2012 from in its GSH control 9 Hashmat et al., Effect of Different pH In buffer pH 7.5 the concentration of GSH carbonate and GSH inside the human at in control is 4.78 µM which has reduced to normal body temperature 37oC. The 2.49 µM after the reaction of lithium remaining GSH concentration at 35oC is carbonate with GSH, shows a sufficient 1.41µM as compare to GSH control reactivity of lithium with GSH at this pH concentration 7.5. temperature-total difference is 1.38µM 2.79 mM at this when lithium carbonate and GSH both Affect of Different temperatures i-e 25 were used in the ratio of 1:1 i-e 0.1mM of degree Celsius, 35 degree Celsius, 45 lithium carbonate (Li2CO3) and 0.1mM of degree GSH (used concentration) Celsius on Li2CO3-GSH and final Interaction in Aqueous Medium concentration in sample mixture left Different human body parts have slightly 3.33µM of each. difference in there temperature but overall temperature can fluctuate about one degree (Fo) throughout the day. Accepted normal human body temperature range is from 36.1oC to 37.2oC. Because of this fact, the effect of Li2CO3 on GSH ( Reduced glutathione) at different temperature (25 degree Celsius, 35 degree Celsius, 45 degree Celsius) in aqueous medium was studied. Our results as shown in table 2 & Fig 3, shows that at 35oC there is greater decrease in the concentration of GSH as compared to carbonate with interaction GSH at of lithium other two temperature i-e 25oC and 45oC. 35oC is a temperature near to normal human body temperature and greater decrease in GSH concentration at this temperature indicates greater chance of interaction of lithium DISCUSSIONS Human body physiological temperature pH of and different compartments of body are very crucial for interaction of almost all medicines taken by human beings for the treatment of different abnormalities ailments/disease including the and under consideration compound of lithium i-e. Li2CO3 which is used for the treatment of mania dipolar episodes and even for the treatment of granulocytopenia and as an adjuvant for cancer chemotherapy (Catan et al, 1977). Lithium carbonate is being used since long for the treatment of above mentioned abnormalities/ diseases and in addition to this still lithium is used for many other disorder, it has also antisucidle Gomal University Journal of Research, 28(1). June, 2012 10 Hashmat et al., Effect of Different pH properties so it was a matter of interest to study the affect of lithium carbonate at metabolism and amphetamine induced locomotor stimulation in rats. Journal of Neural Transmission, P,239-250. different pH buffers as well as at different temperatures and our results show that lithium carbonate-GSH interaction is more at both (pH 7.5) near to body pH and Bymaster F P & Felder C C (2002). Role of cholinergic muscarinic system in bipolar disorder and related mechanism of action of antipsychotic agents. Molecular Psychiatry, 7 (suppl. 1), S57-S63. temperature near to body temperature. As shown in table 1 Fig 2 and table 2 ,Fig 3. CONCLUSIONS Results show that lithium carbonateglutathione interaction is greater at pH 7.5 and temperature 35oC. Our study indicats that lithium is also a cause of considerable depletion in GSH concentration. Although there are so many reasons to used lithium carbonate for the treatment of many human diseases/ abnormalities but this is again a fact that lithium interact with reduced glutathione (GSH) and depletes it. Coles B & Ketterer B(1990). The role of glutathione and glutathione transferases in chemical carcinogenesis: Crit Rev Biochem Mol Biol 25: Pp. 47-70. De Bruin V M S, Marinho M M , & De Sousa, F C F (2000). Behavioural and neurochemical alternations after lithiumpilocarpine administration in young and adult rats. A comparative study. Pharmacology Biochemistry and Behaviour, 65, 574-551. Dringen RGJ & Hirlinger J (2000). Glutatione metabolism in the brain: Eur J Biochem. 267: Pp. 4912-4916. Ellaman GL (1959). Determination of sulfhydryl group Arch. Biochem. Biophys., 89: 70-74. Depletion of GSH may be due to the formation of Li-S-G complex formation or GSSG formation. Li2CO3 +2GSH -1 GS +GS -1 2Li-SG+H2CO3 GSSG REFERENCES Berggren U (1986). Effects of chronic lithium treatment on brain monoamine Ferrie L, Young A H & McQuade R (2005). Effect of chronic lithium and withdrawal from chronic lithium on presynaptic dopamine function in the rat. Journal of psychopharmacology, 19, 229234. Fridovich I (1989). Superoxide dismutases: An adaptation to a paramagnetic gas: J boil. Chem. 264: Pp. 7761-7764. Halliwell B, Gutteridge J M (1986). Oxygen free radicals and iron in relation to biology and medicine: some problems and Gomal University Journal of Research, 28(1). June, 2012 11 Hashmat et al., Effect of Different pH concepts: Arch Biochem Biophys. 246:Pp. 501-514. Hinchman C A & Ballatori N (1994). Glutatione conjugation and conversion to mercapturic acids can occur as a intrahepatic process: J Toxicol Environ Health 41: Pp. 387-409. Jope R S (2001). Lithium selectively potentiates cholinergic activity in rat brain. Progress in Brain Research, 98,317-322. Lenox R H & Manji H K (1995). Lithium. In American psychiatric Association textbook of psychopharmacology (eds A.F. Schatzberg & C.B. Nemeroff), pp. 303349. Lenox R H & Hahn C G (2000). Overview of the mechanism of action of lithium in the brain. Fifty-year update. Journal of Clinical psychiatry, 61 (Suppl. 9), 5-15. Naqui A and Chance B (1986). Reactive oxygen intermediates in biochemistry: Annu Rev Biochem 55: Pp. 137-166. Price L H, Charney D S, Delgado P L(1990). Lithium and serotonin function: implications for the serotonin hypothsis of depression. Psychopharmacology, 100, 3-12. Sechi GDM, Bua G, Satta WM, Deiana GA, Pes GM, & Rosati G (1996). Reduced intervenous glutathione in the treatment of early Pakistan’s disease: Prog Neuropsychopharmacol Biol Psychiatry. 20:Pp. 1159-1170. Shiah I S & yatham L N (2000). Serotonin in mania and in the mechanism of action of mood stabilizers: a review of clinical studies. Bipolar Disorder, 2 77-92 Sohal RS and Weindruch R (1996). Oxidative stress, caloric restriction, and aging Science: (Wash. DC) 273: Pp. 5963. Sundquist A R and Fahey R C (1989). Evolution of antioxidant mechanisms: Thioldependent peroxidases and thioltransferases among prokaryotes: J Mol Evol 29: Pp. 429-435. Wang H, Lu X Q & Zhang Y F (1997). Modulation of phosphatidylinositol turnover on central nicotinic receptors. Zhongguo Yao Li Xue Bao, 18, 341-344. Gomal University Journal of Research, 28(1). June, 2012