Production of diprenylated indole derivatives by tandem incubation
advertisement

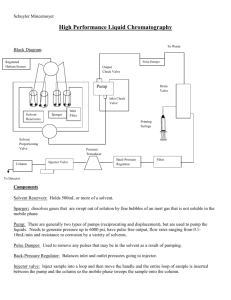
Production of diprenylated indole derivatives by tandem incubation of two recombinant dimethylallyltryptophan synthases Han-Li Ruan, Edyta Stec and Shu-Ming Li* Materials and methods Chemicals Dimethylallyl diphosphate (DMAPP) was prepared according to the method described for geranyl diphosphate by Woodside (1988). Other substrates were obtained from Aldrich, Fluka, Roth, Acros and Alfa Aesar, respectively, and of the highest purity available. Plasmids, bacterial strains and cultural conditions Plasmids pIU18 (Steffan et al. 2007) and pLW40 (Kremer et al. 2007) were used for overproduction of FgaPT2 and 7-DMATS, respectively. Escherichia coli strains XL1 Blue MRF´ and BL21 (DE3) pLysS were used for overexpression experiments and grown in liquid Luria-Bertani medium at 37 °C. Carbenicillin and kanamycin (50 µg·mL -1) were used for selection of recombinant E. coli strains. Preparation of recombinant FgaPT2 and 7-DMATS For preparation of FgaPT2, E. coli BL21 (DE3) pLysS cells harbouring pIU18 were cultivated with kanamycin (50 µg·mL-1) as supplement and induced by 0.5 mM of Isopropyl-β-D-thiogalactopyranoside (IPTG) at room temperature for 6 h. His 8-FgaPT2 was purified with Ni-NTA agarose to homogeneity as judged by SDS-PAGE (Steffan et al. 2007). For preparation of 7-DMATS, E. coli XL1 Blue MRF´ cells harbouring pLW40 were cultivated with carbenicillin (50 µg·mL-1) as supplement and induced by 0.8 mM of IPTG at 37 °C. His 6-7-DMATS was purified with Ni-NTA agarose to homogeneity as judged by SDS-PAGE (Kremer et al. 2007). Conditions for enzymatic reactions Tandem incubations were carried out with 1 mM tryptophan or derivatives, 2 mM DMAPP, 1.7 µM of one of the purified enzymes (FgaPT2 or 7-DMATS) and 10 mM CaCl2 in 50 mM Tris/HCl (pH 7.5) at 37 °C. The reaction mixtures with the first enzyme were incubated first for 16 h. After addition of the second enzyme (1.7 µM) and 2 mM DMAPP, incubation was continued for further 16 h. To terminate the enzymatic reaction, methanol was added to the incubation mixtures, e.g. 100 µl methanol for 100 µl reaction mixture. After removal of the proteins by centrifugation (15,000 x g, 10 min, 4 °C), the enzymatic products in supernatants were analysed on a HPLC system described below. Two independent assays were carried out routinely for quantification. HPLC analysis of enzymatic products Reaction mixtures of FgaPT2 and 7-DMATS were analyzed on an Agilent HPLC Series 1100 by using an Eclipse XBD-C18 column (4.6 x 150 mm, 5 µm, Agilent) at a flow rate of 1 ml min-1. Water (solvent A) and acetonitrile (solvent B), each containing 0.5 % (v/v) trifluoroacetic acid, were used as solvents. A gradient was run from 15 % to 70 % B in A in 15 min. After washing with 100 % B for 5 min, the column was equilibrated with 15 % B in A for 5 min. The substances were detected with a Photo Diode Array detector. For isolation, the same HPLC equipment with a Multospher 120 RP-18 column (250 x 10 mm, 5 µm, C+S Chromatographie Service, Langenfeld, Germany) was used. A linear gradient of 30 - 90 % (v/v) B in 30 min at a flow rate of 3 ml•min-1 was used. The column was then washed with 100 % B for 5 min and equilibrated with 30 % (v/v) B in A for 5 min. Spectroscopic analysis Isolated enzymatic products (50 - 200 µg) were analyzed by 1H-NMR spectroscopy, H-HCOSY on an Avance DRX 500 spectrometer (Bruker) using d6-DMSO as solvent as well as by positive and negative electrospray ionization (ESI) mass spectrometry with a ThermoFinnigan TSQ Quantum. The mass spectrometer was coupled with an Agilent HPLC series 1100 equipped with a RP18-column (2 x 250 mm, 5 µm). For separation, the column was run with 10 % solvent B (methanol) in solvent A (water), followed by a gradient from 10 % to 100 % B over 30 min. After washing with 100 % B for 5 min, the column was equilibrated with 10 % B for 10 min. The flow rate was at 0.2 ml min-1. The NMR data of the isolated compounds are given in Table 2. ESI mass data of 4, 7-di-DMA-L-tryptophan: m/z 341.5 (M+1)+; 339.8 (M-1)ESI mass data of 4, 7-di-DMA-L-abrine: m/z 355.4 (M+1)+; 353.8 (M-1)ESI mass data of 4, 7-di-DMA-11-methyltryptophan: m/z 355.6 (M+1)+; 353.8 (M-1)- Reference List Kremer A, Westrich L, Li S-M (2007) A 7-dimethylallyltryptophan synthase from Aspergillus fumigatus: overproduction, purification and biochemical characterization. Microbiology 153:3409-3416 Steffan N, Unsöld IA, Li S-M (2007) Chemoenzymatic synthesis of prenylated indole derivatives by using a 4-dimethylallyltryptophan synthase from Aspergillus fumigatus. Chembiochem 8:1298-1307 Woodside AB, Huang Z, Poulter CD (1988) Triammonium germanyl diphosphate. Org Synth 66:211-215