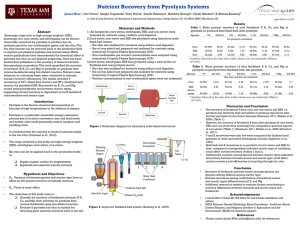
Determination of the temperature at which decomposition
advertisement

Determination of the temperature at which decomposition reactions become exothermic In figure 1, two temperature profiles are presented for an experiment at 0 bar (below) and 5 bar (top) both at 330°C. Three temperatures are presented, one measured just below the grid (supporting the woodchips), one just above the grid (in the bed of woodchips) and one on top of the reactor (above the bed). The gas temperature on top of the reactor is lower than the inlet gas temperature at the first stage of heating. At a specific temperature (the shift temperature), the temperature on top of the reactor starts to rise more rapidly and becomes higher than the inlet gas temperature. This phenomenon can be due to the shift of endothermic pyrolysis at lower temperatures to exothermic pyrolysis at higher temperatures. However, another explanation can be that, at higher temperatures, heat radiation of the reactor wall is more significant leading to a higher heat supply to the reactor bed and thus a higher reactor temperature. When comparing the recordings for 0 bar with 5 bar, a lower shift temperature can be observed at 5 bar. However, this shift temperature is easily affected by deviations from the set heating profile and is therefore not observed as clearly for all experiments. However, a similar trend is present in all other experiments. The average shift temperature attains 310°C (±15°C) at 0 bar and 280°C (±15°C) at 5 bar. At higher heating rates no shift temperature is observer since the temperature on top of the reactor stays below the inlet heating temperature. Streibel et al. [1], reported a shift in the heat of pyrolysis from endothermic to exothermic at 350°C for untreated spruce (heating rate 10°C/min, flow rate 60ml/min). However, CCA promotes the formation of char [2], which is an exothermic process [3]. This may explain the lower shift temperature, observed for CCA treaded wood. Rath et al. [4] reported a shift from endothermic regime to exothermic regime at 409°C for the pyrolysis of spruce (heating rate 10°C/min, flow rate 80ml/min). When the sample was covered with a lid, the shift temperature decreased to 385°C. Covering the sample with a lid, resulted in enhanced exothermic secondary char formation. Similarly, Elevated pressure results in enhancement of secondary char forming reactions, which are exothermic. Therefore elevated pressure results in higher mass retentions and a lowered temperature at which the heat of pyrolysis shifts from endothermic to exothermic. [3, 4] Fig.1. temperature profile as a function of time for an experiment at 5 bar (top) and 0 bar (bellow) both for a residence time of 20min. and a process temperature of 330°C. dotted line: temperature below the grid, dashed line: temperature 1cm above the grid, solid line: temperature on top of the reactor, just above the sample. References [1] T. Streibel, R. Geissler, M. Saraji-Bozorgzad, M. Sklorz, E. Kaisersberger, T. Denner, R. Zimmermann, Evolved gas analysis (EGA) in TG and DSC with single photon ionisation mass spectrometry (SPI-MS): molecular organic signatures from pyrolysis of soft and hard wood, coal, crude oil and ABS polymer, Journal of Thermal Analysis and Calorimetry, 96 (2009) 795-804. [2] L. Helsen, E. Van den Bulck, S. Mullens, J. Mullens, Low-temperature pyrolysis of CCA-treated wood: thermogravimetric analysis, Journal of Analytical and Applied Pyrolysis, 52 (1999) 65-86. [3] W.S.L. Mok, M.J. Antal, effects of pressure on biomass pyrolysis .2. heats of reaction of cellulose pyrolysis, Thermochimica Acta, 68 (1983) 165-186. [4] J. Rath, M.G. Wolfinger, G. Steiner, G. Krammer, F. Barontini, V. Cozzani, Heat of wood pyrolysis, Fuel, 82 (2003) 81-91.