Supplementary Data 1
advertisement

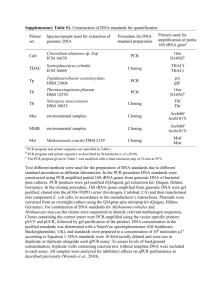
1 SUPPLEMENTARY INFORMATION 2 3 DNA extraction from Freshwater quarry mats 4 DNA was extracted from mat material using the Mo-Bio Power Soil DNA 5 extraction kit, following the protocol for difficult-to-lyse cells (heating tubes to 70°C for 6 10 minutes after addition of solution C1). DNA was quantified by electrophoresis 7 through a 0.7% agarose gel, using 1X TAE buffer, and comparison to a HindIII DNA 8 ladder (New England Biolabs, Ipswich, MA). DNA was stained with ethidium bromide 9 and visualized with a UVP MultiDoc-It Gel Imaging system (UVP, Upland, CA). 10 11 12 PCR amplification and cloning of 16S rRNA genes DNA extraction was followed by PCR using bacteria-specific primers 27F and 13 1492R, based on E. coli numbering (Lane, 1991). PCR amplifications were prepared 14 with 1X Buffer, 0.2 mM each dNTP, 1.5 mM MgCl2 (all from Promega), 0.5 µM of each 15 primer (Integrated DNA Technologies, Coralville, IA), 1.5 U Taq polymerase (New 16 England Biolabs), and ~40 ng DNA template in a final volume of 30 µL. Reactions were 17 performed in a TGradient thermal cycler (Whatman Biometra, Goettingen, Germany). 18 Amplification of 16S rRNA genes from bacteria consisted of 30 cycles of 95C for 30 19 sec, 56C for 30 sec, and 72C for 30 sec with an initial denaturation at 95C for 5 20 minutes and a final extension at 72C for 5 minutes. 21 PCR products were purified with the QIAquick PCR purification kit (Qiagen, 22 Valencia, CA), and were quantified by comparison to a 1 Kb DNA ladder (New England 23 Biolabs) when run on a 1% agarose gel. Cloning of mixed community 16S rRNA gene 24 fragments was performed using the pGEM T-easy vector (Promega, Madison, WI). 25 Ligation into the vector at an insert to vector ratio of 1:1 and transformation using JM109 26 competent cells followed manufacturer’s instructions. 27 28 29 Sequencing and phylogenetic analysis From 32 clones, PCR using M13 vector-specific primers was performed. DNA 30 sequencing reactions were prepared using the forward primer 27f and were sent to the 31 Yale University DNA Analysis Facility. Sequences were edited using the software 32 FinchTV (http://www.geospiza.com/Products/finchtv.shtml). Good quality sequences 33 were used, for a total of 23 clone DNA sequences. Ribosomal RNA gene sequences were 34 searched against the Ribosomal Database Project release 10 (Cole et al., 2009) to 35 determine nearest matches. Multiple sequence alignments were created using the 36 ClustalW interface within the software package BioEdit (Hall, 1999). BioEdit was used 37 to create similarity matrices for all sequences and to determine phylotype groups (based 38 on ≥ 97% similarity). Phylogenetic trees were created with MEGA 5 (Tamura et al., 39 2007), using the minimum evolution method with 1000 bootstrap replicates. Possible 40 chimeras were detected using the Bellerophon server (Huber et al., 2004) and were 41 removed from the analyses. 42 Overall, microbial diversity at this site seemed to be very high; in an assessment 43 of a small subset of 24 clones, none of the sequences were highly similar (>97%) to each 44 other, suggesting that many clones would need to be sampled to gain a full understanding 45 of the diversity within this mat community. The bacteria library, when compared to 16S 46 rRNA gene sequences in the Ribosomal Database Project (Cole et al., 2009), was not 47 clearly dominated by any one phylogenetic group, and few sequences matched strongly 48 with any sequences in the database, despite appearing to be good quality sequences. 49 From the sequence identity matrix generated in BioEdit, aligned and trimmed sequences 50 could be compared to each other and to those selected from the Ribosomal Database. 51 One sequence, clone FW quarry S1, had a 97.5% match with an uncultured 52 Acidobacterium involved in bioremediation of aliphatic hydrocarbons in contaminated 53 soil (Militon et al., 2010). Another clone, L10, matched 97.5% with an uncultured beta 54 proteobacterium from an iron oxidation biofilm in a lagoon in Japan (Sakurai et al., 55 unpublished). Clone L7 matched 98.7% with an uncultured beta proteobacterium from 56 sediments and plankton of a suboxic freshwater pond (Briee et al., 2007). Clone S10 57 matched 98% with an uncultured delta proteobacterium from that same environment 58 (Briee, et al., 2007). Clone S3 matched 99.5% with a deltaproteobacteria sequence from 59 a redox gradient in Baltic Sea sediment (Edlund et al., 2008). Others matched less 60 strongly with Cyanobacteria, Planctomycetes, Geobacter, Verrucomicrobia, or 61 Acidobacteria. S14 matched 81.5% with Sphingobacteria from high mountain lake 62 epilithic biofilms (Bartrons, unpublished). 63 64 65 66 67 68 69 70 Nucleotide sequence accession numbers Partial sequences of ~620 bp were deposited in GenBank (Benson et al., 2011), accession numbers are given in Supplementary Figure 1. 71 REFERENCES 72 73 BARTRONS, M. Bacterial diversity from high mountain lake epilithic biofilms. 74 Unpublished. 75 BENSON D.A., KARSCH-MIZRACHI I., LIPMAN D.J., OSTELL J., and SAYERS E.W. 2011. 76 GenBank: Nucleic Acids Research, v. 39, D32-7. 77 BRIEE, C., MOREIRA, D., and LOPEZ-GARCIA, P. 2007. Archaeal and bacterial community 78 composition of sediment and plankton from a suboxic freshwater pond: Res. Microbiol. 79 v. 158, p. 213-227. 80 COLE, J.R., WANG, Q., CARDENAS, E., FISH, J., CHAI, B., FARRIS, R.J., KULAM-SYED- 81 MOHIDEEN, A.S., MCGARRELL, D.M., MARSH, T., GARRITY, G.M., AND TIEDJE, J.M. 2009 82 The Ribosomal Database Project: improved alignments and new tools for rRNA analysis: 83 Nucleic Acids Res., v. 37 (Database issue), D141-D145. 84 EDLUND, A., HARDEMAN, F., JANSSON, J.K., and SJOLING, S., 2008, Active bacterial 85 community structure along vertical redox gradients in Baltic Sea sediment: Environ. 86 Microbiol., v. 10, p. 2051-2063. 87 HALL, T.A. 1999. BioEdit: a user-friendly biological sequence alignment editor and 88 analysis program for Windows: Nucleic Acids Symposium Series, v. 41, p. 95-98. 89 HUBER, T., FAULKNER, G. AND HUGENHOLTZ, P. 2004. Bellerophon: a program to detect 90 chimeric sequences in multiple sequence alignments: Bioinformatics, v. 20, p. 2317- 91 2319. 92 LANE, D.J. 1991. 16S/23S rRNA Sequencing: Nucleic Acid Techniques in Bacterial 93 Systematics, in, Stackebrandt, E. and Goodfellow, M., (eds), pp. 115-175. John Wiley 94 and Sons, New York. 95 MILITON C., BOUCHER D., VACHELARD C., PERCHET G., BARRA V., TROQUET, J., 96 PEYRETAILLADE E., and PEYRET, P., 2010, Bacterial community changes during 97 bioremediation of aliphatic hydrocarbon-contaminated soil: FEMS Microbiology 98 Ecology, v. 74, p. 669-681. 99 SAKURAI, K., TAZAKI, K., and YAMAGUCHI, K. Identification of bacteria in an iron- 100 oxidation biofilm at Shibayama lagoon, Ishikawa, Japan. Unpublished. 101 TAMURA, K., PETERSON, D., PETERSON, N., STECHER, G., NEI, M., AND KUMAR, S. 2011. 102 MEGA5: Molecular Evolutionary Genetics Analysis using Maximum Likelihood, 103 Evolutionary Distance, and Maximum Parsimony Methods: Molecular Biology and 104 Evolution, v. 28, p. 2731-2739.