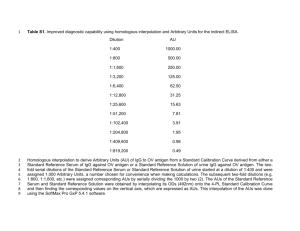
Antibody are immunoglobulins that react specifically with the antigen
advertisement

Antibody or Immunogloblin molecule.
Antibody are immunoglobulins that react specifically with the antigen that
stimulated their production. They make up to 20% of plasma proteins and were
initially detected by analytic techniques, such as electrophoresis, in the
gammaglobulin fraction of serum.
All antibodies have the same basic molecular structure. They made up of light (L)
and heavy (H) chains, which refer to their relative molecular weights; the light
chains have a molecular weight of approximately 25,000 and the heavy chains
have a molecular weight of approximately 50,000 to 70,000. In the basic
immunoglobulin molecule, there are two heavy and two light chains linked
together by intermolecular disulfides bonds.
Immunoglobulins
Antigen binding sites
Amino terminal
Constant
region
{
} Variable region
Light Chain
Heavy Chain
Carboxyl terminal
There are five different classes of human heavy chain with slight different
structures. These are µ for IgM, δ for IgD, γ for IgG, ε for IgE and α IgA.
The light chains are divided into two type, қ (kappa) or λ (lambda). Both types of
light chains are found in all five classes of immunoglobulin, but only one
antibody contains only one type of light chain.
Any one of IgG molecule consists of identical H chains and identical L chains
organized into Y-shaped structure. The IgG class can be divided into for
subclasses include IgG1 to IgG4. The structures of aminoacid sequences of
members of two different subclasses e.g IgG2 and IgG3 are more similar to each
other than the structures of two immunoglobulins from different classes e.g IgG
and IgA.
The basic immunoglobiln contains molecular parts with different functions. If the
basic immunoglobulin molecule (IgG) is subjected to proteolytic cleavage, several
fragments are produced. For example, if the enzyme papin is used to cleave IgG,
two major types of fragment are obtained. One fragment bind antigen and is
referred to as Fab (fragment antigen-binding). The other fragment, known as Fc
(fraction crystallizable), does not bind antigen but activate the complement
pathway and has various biologic effectors function such as the ability to bind to
receptor and macrophages and various other cells. If the proteolytic enzyme
pepsin is used, the two Fab fragments remain linked F(ab-)2 but the Fc fragment
is digested to small fragment and effector functions are lost. These finding are
lost. These suggest that the different molecular parts of the Immunoglobulin
molecule have different functions. One for binding antigen and responsible for
other biological effectors functions.
The amino acid sequence of both H & L chain can be differentiated into regions
that are highly variable in sequence (VL and VH) and regions that are essentially
constant (CL and CH). The C regions carry out the biological effector functions
such as the ability to bind complement proteins and the V region bind antigen.
The variable regions are critical to respond to a large numbers of different antigen
structures.
Additional 3D-struture determination has revealed that the Immunoglobulin are
composed of folded, repeating segments called domains. The light chain consist
of one variable domain and one constant domain. The heavy chain consist of one
variable and three or more constant domain. Each domain is approximately 110
amino acid residues long.
Features and Biological properties of Immunoglobulin Classes.
Antibodies can occur as soluble protein in the circulation or be display on the surface
of B cells.
The primary function of all antibodies is to bind antigen. This can result in the
inactivation of a pathogen by agglutination for example and thus preventing their
entry to host cells.
If bacteria are coated with antibody (opsonization), they will be engulfed by
phagocytic cells or activate complement system which initiate a lytic reaction that
destroys the pathogenic organism.
The five classes of antibody have different function that are a consequent of
differences in structure.
IgM. This is the predominant antibody early in an immune response. It has a
pentameric structure, composed of five identical heavy and five identical light chain,
held together by a joining J chain. The H-chains of IgM differ from those of IgG in
having four constant domains instead of three in IgG. It has 10 antigen-binding sites
and because of this it is the most efficient antibody at agglutinating bacteria and
activating complement.
IgM molecule
IgD. IgD generally has a low serum concentration and is unstable in serum, being
quickly degraded by serum plasmin. . IgD is found on the surface of B cells as a
receptor molecule . IgD function is not clear, but it may involved in B-cell
development.
IgG. This is the most antibody molecule in serum. It also present in the sera for long
time, it is able to cross the placenta to allow maternal protection of the newborn.
There are 4 subclasses of human IgG (IgG1-IgG4) and each of these has slightly
different properties. For example IgG2 is generally the antibody subclass found
predominate in response against polysaccharide antigen of encapsulated bacteria.
IgG and subtypes
IgE. Has a high MW 72 000, and it has five constant domains (one variable and 4
constant domain). It is present at the lowest concentration of all antibody classes
in serum.It is found in serum, is found on the surface of basophiles and mast cells
It Play a role in immunity to parasites and It associated with allergic disease
(asthma).
Binding of antigen to IgE coupled to an Fc receptor on mast cells and basophils
triggers an allergic reaction by activation of the mast cells and release of histamine.
IgA. Is present in the serum and is also the main class of Immunoglobulin found in
various secretions such as saliva, milk and tears and is heavily represent in the
mucosal, epithelia of respiratory, genital and intestinal tract.
The two subclasses of IgA in human, IgA1 and IgA2 appear to have the same
function. IgA has different structure depending on whether it is in serum or secretions.
In serum IgA adopts the basic 2 heavy and 2 light chain. IgA in secretions (sIgA)
consists of two molecules of IgA, a joining J chain and one molecule of additional
protein known as secretory piece. The function of this secretory piece appears to
protect the molecule from proteolytic attack and to facilitate its transfer across
epithelial cells into secretion. The secretory piece is not actually made by the antibody
molecule but it added to the IgA in a special way.
IgA secreted by plasma cells in the mucosa binds to a receptor, called poly-Ig receptor
on mucosal epithelial cells. The poly-Ig receptor, together with the bound IgA is
internalized by the epithelial cells and the poly-Ig receptor is cleaved. Finally the IgA
is secreted into the lumen with part of the poly-Ig receptor which is known as the
secretory piece and attached to the IgA molecule.