Glycosides
advertisement

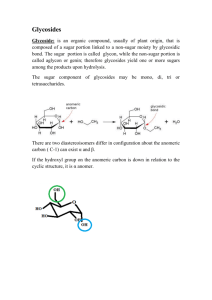
1 Glycosides Definition: Glycosides are (usually) non-reducing compounds, on hydrolysis by reagents or enzymes yield one or more reducing sugars among the products of hydrolysis. non-sugar (genin) glycosidic linkage sugar (glycone) 1- Alcoholic or phenolic (aglycone): e.g., O-Glycoside CH2 OH CH2OH O-C6 H11 O5 OH C6 H12 O6 + -H2O Glycosidic linkage Sugar Salicin 2- Sulphur containing compounds: e.g., S-Glycoside Glycosidic linkage SH C6H12O6 Sugar + CH2 CH CH2 C CH2 N CH CH2 OSO3 K Sinigrin S C6 H11 O5 N OSO3 K C 2 3- Nitrogen containing compounds: e.g., N-Glycoside NH2 OHCH2 NH2 OH O H + H N N N OH OH N H N N N OHCH2 N O Glycosidic linkage H H OH OH Adenine 4- C-Glycoside HO C6 H12 O6 O HO OH O OH + CH2 OH CH2 OH Glycosidic linkage C6 H11 O5 Barbaloin 1- Sugars exist in isomeric α and β forms. Both α and β Glycosides are theoretically possible. 2- All natural glycosides are of the β Type. 3- Some α linkage exists in sucrose, glycogen and starch. Also the glycoside K-strophanthoside (strophanthidin-linke to strophanthotriose (Cymarose + β-glucose + α- glucose). 3 1- According to the type of glycosidic linkage: α- glycoside (α-sugar) and β-glycosides (β-sugar). 2- According to the chemical group of the aglycone involved into the acetal union: a. O-glycoside (OH group) b. S-glycoside (SH group). c. N-glycoside (NH group). d. C-glycoside (C group). 3- According to the nature of the simple sugar component of the glycoside: a. Glucosides (the glycone is glucose). b. Galacosides (the glycone is galacose). c. Mannosides (the glycone is mannose). d. Arabinosides (the glycone is arabinose). 4- According to the number of the monosaccharides in the sugar moiety: a. Monoside (one monosaccharide) e.g., salicin. b. Biosides (two monosaccharide) e.g., gentobioside. c. Triosides (three monosaccharide) e.g., strophanthotriose. 5- According to the physiological or pharmacological activity ‘therapeutic classification) a. Laxative glsycosides. 4 b. Cardiotonic glycosides. 6- according to the correlation to the parent natural glycoside: a. primary glycosides e.g., amygdalin, purpurea glycoside A, b. Secondary glycosides e.g., prunasin, digitoxin. 7- According to the plant families. 8- According to the chemical nature of the aglycone: a. Alcoholic and phenolic glycosides (aglycones are alcohols or phenols) b. Aldehydic G (aglycones are aldehydes). c. Cyanogenic G (aglycones are nitriles or derivatives of hydrocyanic acid). d. Anthracene or anthraquinone G (aglycones are anthracene der.). e. Steroidal G (aglycones are steroidal in nature, derived from cyclopentanoperhydrophenanthrene) . f. Coumarin G (aglycones are derivative of benzo α-pyrone). g. Chromone glycosides (aglycones are derivatives of benzoδ-pyrone) h. Flavonoidal G (aglycones are 2-phenyl chromone structure). i. Sulphur containing or thioglycosides (aglycones are contain sulphur). j. Alkaloidal glycosides (aglycone is alkaloidal in nature) e.g., glucoalkaloids of solanum species. 5 Sugars in glycosides: 1- Monosaccharide (glucose in salicin, rhamnose in ouabain) 2- Disaccharides (gentiobiose in amygdalin). 3- Trisaccharides (strophanthotriose). 4- Tetrasaccharides (purpurea glycosides) 5- Rare sugers (deoxy sugers) 6- Sugar linked in one position to the aglycone rarely in 2 positions as sennosides. A- 6-deoxy sugars e.g., 1- methylpentoses CHO H C OH H C OH HO C H HO C H CH3 2- α-L-rhamnose. O OH HO CH3 OH OH 6 B- 2,6-deoxy sugars (called rare sugars) e.g., 1- D.digitoxose 2- D.cymarose 3- diginose CHO CHO CHO C H2 C H2 C H2 C OH C OCH3 C OH C OH C OH C OH CH3 CH3 H3 CO C HO C H C OH CH3 C- 2-deoxy sugars e.g., 2-deoxy-D-ribose HOH2 C H O H OH OH H Characteristic of 2-deoxy sugers: 1- Give positive Schiff’s test for aldehydes. 2- Positive Keller-Kelliani test. Diversity in structure makes it difficult to find general physical and chemical properties: 7 1- A- Most glycosides are water soluble and soluble in alcohols. B- Either insoluble or less soluble in non polar organic solvents. C- More sugar units in a glycoside lead to more soluble in polar solvents. 2- Glycosides do not reduce Fehling’s solution, but when are susceptible to hydrolysis give reducing sugars (C-glycosides are exceptions). 1- Acid hydrolysis: a- Acetal linkage between the aglycon and glycone more unstable than that between two individual sugars within the molecule. b- all glycosides are hydrolysable by acids non specific (except Cglycosides). c- Glycosides containing 2-deoxy sugars are more unstable towards acid hydrolysis even at room temperature. d- C-glycosides are very stable (need oxidative hydrolysis). 2- Alkali hydrolysis: 1- mild alkali 2- strong alkali 3- Enzyme hydrolysis: 1- Enzymatic hydrolysis is specific for each glycoside there is a specific enzyme that exerts a hydrolytic action on it. 8 2- The same enzyme is capable to hydrolyze different glycosides, but α and β sterio-isomers of the same glycoside are usually not hydrolysed by the same enzyme. 3- Emulsin is found to hydrolysed most β-glycoside linkages, those glycoside are attacked by emulsin are regarded as β-glycosides. 4- Maltase and invertase are α-glycosidases, capable of hydrolyzing αglycosides only. 1- Water mixed with different proportions of methanol or ethanol (most suitable extracting solvent). 2- Non-polar organic solvents are generally used for de-fating process. 3- Glycosides are not precipitate from aqueous solutions by lead acetate. 1- Destruction of hydrolysing enzymes. a. Drying for 15-30 min. at 100 C˚. b. Place plant in boiling water or alcohol 10-20 min. c. Boiling with acetone. d. Cold acid pH treatment. e. Extract at very low temperature. 2- De-fating or purification of the plant material (in case of seeds). 3- Extraction of the glycosidal constituents by alcohol, water or dilute alcohols. Some times ether saturated with water for dry material. 9 4- Concentrate the alcoholic extract (to get rid of the organic solvent). Add water (or hot water)→ filter any precipitate. 5- Purify aqueous extract: a- Extract non glycosidal impurities by org solvent. b- Water soluble impurities precipitate by lead acetate. 6- Precipitate excess lead salts. 7- Isolation of the glycosides from the purified aqueous solution, by crystallization. They do not themselves reduce Fehling’s. but reducing sugars upon hydrolysis. To test for the presence of glycosides Estimate reducing sugars before and after hydrolysis. (by acids or enzymes) 1- Steroidal or cardiac glycosides: Give positive Liebermann’s test (steroidal structure). 2- Anthraquinone glycosides and/or aglycone: Give positive Borntrager’s test, characteristic reddish coloration with alkalies. 10 3- Flavonoidal glycosides and/or aglycones: Characteristic color with, NH4OH, AlCl3, FeCl3. 4- Cyanogenetic glycosides give upon hydrolysis hydrocyanic acid can be easily tested by change Na bikrate paper (yellow) to red color. 5- Sulphur containing glycosides give black precipitate of silver sulphate upon treatment with AgNO3 solution. 1- Keller Killiani’s test for 2-deoxy sugers: Specificity of action of the hydrolyzing enzymes is often applied for the identification of the sugar moieties of glycosides or even the glycoside as alcohol. 1- Scillarin A [acid hydrolysis] →→→ Scillaridine A + Scillabiose Scillabiose [Scillabiase] →→→ Rhamnose + glucose. CHO 2- Prunasin [Prunase] →→→ glucose + HCN + H O C 6 H 11 O 5 C CN 3- Amygdalin [amygdalase] → Prunasin + glucose 4- Myrosin enzyme is specific for thio D- glucosides e.g., Sinigrin and sinalbin. 11 Determination of the glycosidic linkages: 1- By the use of α and β glycosidases. 2- By acid hydrolysis of glycosides, immediate optical activity measurement of the resulting solution. Color reactions based on the sugar moiety [2-deoxy sugars]: 1- Keller Killiani: glacialacetic acid containing + FeCl3 + H2SO4 → brown ring free from red (acetic acid a quire blue). 2- Xanthydrol: xanthydrol in glacial acetic containing 1% HCl + glycoside [heat]→ red color. N.B. Stability indicating after extraction. U.S.P. Medicinal importance of glycosides: 1- Cardiac drugs: cardiotonic glycosides e.g., digitalis glycosides, strophanthus, squill. 2- Laxatives e.g., anthraquinone glycosides of senna, aloes, rhubarb, cascara, frangula. 3- Counter irritants e.g., thioglycosides and their hydrolytic products ‘allylisothiocyanate’ 4- Analgesics e.g., methylsalicylate ‘a hydrolytic product of gaultherin. 5- Anti rheumatic e.g., salicin. 12 6- Some glycosides are claimed to reduce the capillary fragility e.g., flavonoidal glycosides, rutin, hisperidin. 7- Anti-inflamatory: e.g., the glycoside glycyrrhizin has a demulcent, expectorant and antispasmodic action. 8- More recently as an anticancer agent e.g., amygdalin known in the U.S. as Laetrile. 13 1-The genins of all cardiac glycosides are steroidal in nature, that act as cardiotonic agents. 2-They are characterized by their highly specific action cardiac muscle, increasing tone, excitability and contractility of this muscle, thus allowing the weakened heart to function more efficiently. Lactone ring 12 11 1 2 R O 9 4 16 14 8 10 5 17 13 3 Sugar CH3 OH 15 7 6 All cardio active glycosides are characterized by the following structural features: 1- The presence of β-OH at position C-3, which is always involved in a glycosidic linkage to a mono, di, tri, OR tetra saccharide. 2- The presence of another β-OH group at C-14. 3- The presence of unsaturated 5 or 6- membered lactone ring at position C-17, also in the β configuration. 4- The A/B ring junction is usually (cis), while the B/C ring junction is always (trans) and the C/D ring junction is in all cases (cis). 5- Additional OH groups may be present at C-5, C-11 and C-16. 14 1- Cardiac glycosides that α-β unsaturated 5-membered lactose ring in position C-17 are known as cardenolides. These are represented by the digitalis and straphanthus group. 2- Digitalis glycosides contain angular methyl group at C-10, while strophanthus glycoside are characterized by presence of either an aldehydic (CHO) or primary alcoholic (C`H2OH) group at C-10. O OH 12 11 1 2 O 5 4 14 8 10 3 Sugar 17 13 9 R CH3 O OH 16 15 7 6 Cardenolides Digitalis glycosides R=CH3 Strophanthus glycosides R=CHO OR CH2OH 3- Cardiac agents that have doubly unsaturated 6-membered lactone ring in position C-17 are referred to as Bufadienolides. 4- This group includes the squill glycosides and the toad venom, Bufotoxin. 15 O O OH 12 11 1 2 13 9 O 5 4 15 8 10 16 14 3 Sugar 17 R1 7 R2 6 Bufadienolides Squill glycosides Bufotoxin R1=OH, R2=H R1 & R2 = ester group 5- The glycone portion at position C-3 of cardiac glycosides may contain four monosaccharide molecules linked in series. Thus, from a single genin one may have a monoside, a bioside, a trioside or a tetroside. 6- With the exception of D-glucose and L-rhamnose, all the other sugars that are found in cardiac glycosides are uncommon deoxy-sugars e.g., Digitoxose, Cymarose, Thevetose. 16 CHO CHO C H2 C H2 C OH C OCH3 CHO C OH C OH C OH C OH CH3 O C H H C OH H C OH CH3 CH3 Cyamarose Thevetose CH3 Digitoxose HC OH Isolation difficulties: 1- Major difficulty in the isolation of 1ry glycosides from the crude drug.. why? because 1ry glycosides are converted into secondary glycosides by hydrolysable enzymes. 2- Other difficulty is the existence of several closely related glycosides in the same drug, which are extremely difficult to separate and purify. Origin: D. purpurea, D. lanata, D. lutea and D. thapsi The structures of the common aglycones of the digitalis group are indicated below: 17 O O R1 12 11 1 2 16 9 14 8 10 3 H Compounds O 5 4 17 13 OH R2 15 7 6 R1 R2 Digitoxigenin H H Gitoxigenin H OH Digoxigenin OH H DX = Digitoxose, DX (AC)=Acetyldigitoxose,G = Glucose. 1- Glycosides derived from Digitoxigenin: a- Lanatoside A = Digitoxigenin---DX---DX----DX(AC)---G. b- Acetyl-digitoxin = Digitoxigenin---DX---DX----DX---(AC). c- Digitoxin = Digitoxigenin------DX---DX----DX. d- Purpurea gly A = Digitoxigenin---DX---DX----DX---G 2- Glycosides derived from Gitoxigenin: a- Lanatoside B = Gitoxigenin---DX---DX----DX(AC)---G. b- Acetyl-gitoxin = Gitoxigenin---DX---DX----DX---(AC). c- Gitoxin = Gitoxigenin------DX---DX----DX. d- Purpurea gly B = Gitoxigenin---DX---DX----DX---G 18 3- Glycosides derived from Digoxigenin: a- Lanatoside C = Digoxigenin---DX---DX----DX(AC)---G. b- Acetyl-digoxin = Digoxigenin---DX---DX----DX---(AC). c- Digoxin = Digoxigenin------DX---DX----DX. d- Deslanoside = Digoxigenin---DX---DX----DX---G 1- The 1ry glycosides Lanatoside A, Lanatoside B, Lanatoside C are acted by specific enzyme which split the terminal acetyldigitoxin, glucose, give acetylgitoxin the and 2ry glycosides acetyldigoxin respectively. 2- The deacetyl-lanatosides A, B and C can be obtained by the alkaline hydrolysis of the corresponding lanatosides. 3- Digitoxin, gitoxin and digoxin are obtained by the action of alkali on their acetyl-derivatives. Lanatoside A purpurea gly. A Specific enzyme Alkaline hydrolysis Specific enzyme Digitoxin Acetyldigitoxin Alkaline hydrolysis Acid hydrolysis Digitoxigenin + 3 digitoxose 19 1- The glycoside K-strophanthoside (a trioside), K- strophanthin B (bioside) and cymarin (a monoside) were isolated from different strophanthus species. 2- The 1ry glycoside K-strophanthoside gives by hydrolysis one molecule of glucose and the 2ry glycoside K- strophanthoside B or K- strophanthin B. 3- The later gives by hydrolysis one molecule of glucose and the tertiary glycoside cymarin, which on turn hydrolyze into the genin K-strophanthidin and the deoxysugar cymarose. O 11 1 2 O Cymarose B-glucose a-glucose 4 OH 6 17 K- strophanthidin 13 16 CHO 9 10 5 3 12 O 14 8 OH 7 15 Cymarin K- strophanthin B K- strophanthoside 20 The seeds of Strophanthus gratus contains another glycoside named Ouabain or (G-strophanthin), which yield on hydrolysis rhamnose and the aglycone ouabagenin. Ouabagenin differs from K-strophanthidin in having 2 additional (OH) groups at C-1 and C-11 and having a 1ry alcoholic group at C-10 instead of the aldehydic group. O O OH OH OH 1 2 11 CH2 9 O 5 4 17 13 16 14 8 10 3 Rhamnose 12 OH 15 7 6 OH Ouabain (G-strophanthin) This group of cardioactive agents includes the squill glycosides (the scillarins) and the Toad poison (Bufotoxin). 21 The genins of squill glycosides differ from those of the cardenolides in two important aspects: 1- They have six membered doubly unsaturated lactone ring in position C-17. 2- They have at least one double bond in the steroid nucleus. O O OH Glucose-Glucose-Rhamnose O Scillaridin A Proscillaridin A Scillarin A Glucoscillarin A The Bufadienolides of Squill Name of glycosides Structure Glucoscillarin Scillaridin A ---RH—G---G Scillarin A Scillaridin A ---RH—G Proscillaridin A Scillaridin A ---RH 22 * The different cardiac glycosides show different solubilities in aqueous and organic solvents. They are usually soluble in water or aqueous alcohol and insoluble in the fat solvents with exception of chloroform and ethylacetate. * The higher number of sugar units in the molecule, the greater solubility in water but lower soluble in chloroform. * Alcohols are good solvents for both the glycosides and the aglycones. Therefore, they are considered as the solvents of choice for the extraction of all CG from drugs. * pet.ether and ether are used for defatting process of drug, they do not dissolve CG. 1- Acid hydrolysis cleavage of the glycosides into aglycones and sugar residues. 23 2- Specific enzyme usually coexist with CG in plants, which may split the primary G into G with less sugar units. Thus, CG deteriorate during drying and storage unless special precautions are taken. 3- So it is required by many pharmacopoeias that CG containing drugs must contain not more than specified moisture content and that these drugs should be stored in sealed containers over dehydrating agents. 4- It is recommended to heat stabilize these CG, by destroying the enzymes at higher temperatures. At higher temperature, the tertiary OH gp at C-14 may split off as water, leading to formation of an inactive anhydro-form of CG. O 12 11 1 R 2 9 O 8 10 5 4 14 OH 11 16 -H2O 1 15 2 R Sugar O 5 16 9 14 15 8 10 4 17 13 3 7 6 CH3 12 17 13 3 Sugar CH3 O O 7 6 5- The gitoxin has in addition to tertiary OH at C-14 another secondary OH at C-16. Both OH gps split as water by the action of H2SO4 with the formation of two additional double bonds. These with the double bond of the lactone ring from a O 24 conjugated double bond system that makes the compound fluorescent in UV light. O 12 11 1 2 R 9 O 8 10 5 4 14 11 OH 16 OH -2H2 O 1 15 2 R Sugar O 5 16 9 14 15 8 10 4 7 6 The detection of gitoxin in other digitalis G is based on the above mentioned reaction. 1- CGs are steroidal in nature, give +Ve with Liebermann’s and Salkoviski’s test. 2- CG that contain deoxy-sugars are distinguished by Keller Kiliani’s test, e.g., digitoxose and cymarose. 3- Cardenolides are distinguished from the scillarins by a group of color reagents, that are all alkaline solutions of aromatic nitro compounds, namely, 17 13 3 7 6 CH3 12 17 13 3 Sugar CH3 O O O 25 Kedde’s reagent, 3,5 dinitrobenzoic, Raymond’s reagent, metadinitrobenzene, Baljet’s reagent, picric acid, Legal’s test, alkaline solution of sodium nitroprusside. 4- All these nitrocompounds react with the active methylene of the five membered lactone ring (in alkaline medium) to give characteristic colors. 1- Cardiotonics, CHF, rheumatic heart disease, atherosclerosis, HTN. 2- Diuretics (capillary of the kidneys are dialated). 1- The glycone part displays a great influence on the solubility and the rate of absorption and distribution of the glycosides to the site of action. 2- Small change in the molecules such as a change of the location of the OH gp, modify the cardiac activity or even eliminate it completely. 26 3- The saturation and/or cleavage of the lactone ring, destroys the cardiac activity. Therefore, the closely related CG, differ greatly in the rate of absorption, duration of action and their cumulative effect. 1- digitalis leaf (digitalis tablets) 2- digitoxin tablets 200μg/tablet 3- digoxin injection contain 0.0025% digoxin 4- digoxin tablets contain 250μg/tablet 5- gitalin, lanatoside C, deslanoside, strophanthin, ouabain and squill. strophanthus, 27 Anthraquinone Anthrone O Anthranol O 1 8 OH 1 8 7 9 2 4H 7 9 2 6 10 3 6 10 3 5 5 4 H H 4 O H O 2H 1 8 7 9 2 6 10 3 5 H OH 4 Oxanthrone 1- O-glycosides where the aglycone moiety is 1,8 dihydroxyanthraquinone derivatives, e.g., Gl O O 8 9 OH 1 2 10 5 4 O Aloe-emodin-8-glycoside Gl O O 8 9 OH 1 2 10 CH2 OH 5 Gl O O 8 9 O Rhein-8-glycoside 2 10 COOH 4 OH 1 5 CH3 4 O Chrysophanol-8-glycoside 28 2- O-glycoside where the aglycone moiety partially reduced 1,8 dihydroxy anthraquinone, e.g., Oxanthrone-type. Gl OH H 1 9 8 7 OH O 6 2 3 10 5 4 O Emodin-oxanthrone-9-glucoside 3- C-glycoside where the aglycone structure (anthrone der.) O OH 8 7 6 5 OH 9 1 2 10 4 3 H CH2 OH C6 H11 O5 Barbaloin 4- O-glycosides where the aglycone moiety is di-anthrone der. (i.e., dimmer) e.g., Sennosides where there is C-C bridge between the anthranol units. Sennoside A&B Gl 7 O O 8 6 5 OH 9 1 2 10 4 3 H COOH H COOH Gl O O OH 29 The most widely used drugs that contain anthracene compounds are: Consists of the dried leaflet of Alexandrian or Khartoum senna, Cassia senna (C.acutifolia), Tinnevelly senna (C.angustifolia). Constituents: Dimeric anthracene glycosides derived from two anthrones moieties which may be: OH 8 O OH 1 9 OH 2 10 5 4 Aloe-emodin anthrone O OH 1 9 8 CH2 OH 2 10 5 COOH 4 Rhein anthrone 1- Similar anthrone moiety (Homo-dianthrones) i.e., 2 rhein anthrone moieties condensate through two C-10 atomes. Thus it can be exist in two optical forms, Sennoside A (Lform) & Sennoside B (meso form). 30 Gl 7 O O 8 6 5 OH 9 1 2 10 4 3 COOH H H COOH Gl O OH O Sennosides A &B 2- Or different (Hetero-dianthrones) i.e., one rhein-anthrone & one emodin anthrone, Sennoside C (L- form) and Sennoside D (meso form). Gl 7 O O 8 6 5 OH 9 1 2 10 4 3 H CH2 OH H COOH Gl O O OH Sennoside C&D 31 The dried bark of Rhamnus purshiana Family Rhamnaceae. B. P. specified that the collection must be made at least one year before the bark is used (fresh bark contains an emetic principle). Constituents: A- Four primary glycosides: 1- cascarosides A&B (glycosides of barbaloin) 2- cascarosides C&D (glycosides of chrysaloin) O OH H OH Gl O O CH2 OH Gl CH2 OH H Gl Barbaloin O OH H OH Cascaroside A& B OH Gl CH3 Gl Chrysaloin O O H OH CH3 Gl Cascaroside C & D B-Two aloins (secondary glycosides): Barbaloin derived from (C-10-C-glycoside) of aloe-emodin anthrone and chrysaloin derived from (C-10-C-glycoside) of chrysophanol anthrone. 32 C- A number of O- glycosides: e.g., derived from emodin, emodine oxanthrone, aloe emodin and chrysophanol. OH O OH O OH OH CH2 OH CH3 O O Aloe emodin Chrysophanol E- Free anthraquinones: Aloe emodin, chysophanol and emodin. 1- Frangulin (frangula emodin rhamnoside). 2- Glucofrangulin (frangula emodin glucorhamnoside). OH O RO OH CH3 O Frangulin Glucofrangulin R= Rhamnose R= Rhamnose-glucose 33 3- hydrolysis of glucofrangulin yields frangulin and glucose. 4- Hydrolysis of frangulin gives frangula emodin and rhamnose. 1- Consist of glycoside of rhein, rhein anthrone, chrysophanol and aloe emodin. 2- Dianthrones of heteroanthrone types are palmidin A, B, C, Rheidins, sennosides A&B and their oxalate esters (sennosides E&F). 3- The presence of tannins in rhubarb makes the drug constipating. So in small doses, rhubarb exerts no purgative action but acts only as intestinal astringent, but large doses cause purgation. Cascara is a purgative, mainly in the form of liquid extract, elixir or as tablets prepared from a dry extract. The laxative action of the crude drugs is always higher than from their content of anthracene der. The different 34 anthracene der. contained by the crude drug are said to exert a synergistic action. Thus, the naturally occurring anthracene glycosides were found superior to the synthesis of numerous hydroxyl anthracene der. Some of these synthetic compounds act too drastically and also caused kidney damage. The only compound which is used to some extent in current medicine is danthrone. It is also used as a standared in colorimetric assays of anthraquinone glycosides. OH O OH O Danthrone Note: 1- The 1ry glycosides are more active than the aloins while the free anthraquinon have little purgative activity. 2- C-C glycosides, aloins are very resistance to hydrolysis and are not easily hydrolysed (like other anthrones and anthranols) to corresponding anthraquinones. 35 3- Aloin type glycosides are present in aloes and other anthracene bearing drugs of the family liliaceae. 1- Glycosilation: The purgative action of anthracene bearing drugs is owed to their anthracene glycosidal content rather than their content of free anthracene aglycones (i.e., glycosylation is the main requirement for activity, as the sugar moiety serve to transport the aglycone to the site of action in the large intestine). 2- Hydroxylation: Hydroxylation of C-1, C-8 is essential for activity. Increase hydroxylation leading to increase solubility. 3- Oxidation level: The degree of oxidation at positions C-9 & C-10 plays an important role in the pharmacological activity. Higher oxidation level at C-9 & C-10 caused lowering of activity. i.e., anthrones and anthranols are more potent than their corresponding oxanthrones, which in turn more active than their corresponding anthraquinones. Complete reduction of C-10 &C-9 lead to complete loss of activity. 36 4- The nature of substances at C-3: Derivative with CH2OH (as in aloe emodin) are more active than those with CH3 substitution. The latter more active than derivative with COOH substitution at C-3. Anthraquinone glycosides containing adimer more active than a monomer. 5- Effect of storage on the active of anthracene glycosides: a- Prolonged storage of anthracene bearing drugs may bring oxidation of anthranols and anthrones to give the less active anthraquinones. Thus, the activity of drugs decreases by time. However, anthraquinone glycosides do not cause any griping action (like anthranol and anthone), thus no antispasmodic such as belladonna is prescribed with them. b- Drugs as senna, Aloe and cascara preparations retain their activity for a long time. c- Cascara and frangula must be aged for one year before it is used for medicinal preparation.WHY? 37 Stability is achieved as follows: 1- In senna, there is dimeric glycoside in which a C-C bridge between two anthrone units is formed (the C-10 position of one anthrone is involved in a C-C-covalent bonding with C10 of the other anthrone). Thus, the C-10 position can not be easily oxidized and the anthrone structure is stabilized. 2- In the aloe, the aloins (barbaloin & chrysaloin) contain CC glycosidic linkage (anhydroglycosides) stabilise the anthrone structure. 4- In cascara, cascarosides have an additional O-glycosidic linkage (beside the C-10-C glycosidic linkage. The solubility of cascarosides is increased and thus, produce higher pharmacological activity. The glycosides are extracted and hydrolyzed by boiling the drug with acids. The aglycones are extracted from the acidic solution with ether or benzene. Upon shaking the ether or benzene layer with aqueous alkali or ammonia solution, the aqueous layer assumes a deep red color, because of the formation of anthraquinone salts. 38 Borntrager’s reaction can distinguish anthraquinones from anthrones and anthranols which do not give the test unless they are converted to anthraquinone by oxidation with mild oxidants such as hydrogen peroxide or ferric chloride. Official anthraquinone drugs in B.P and U.S.P.: 1- Senna leaf & senna fruit (pod). 2- Aloes. 3- Cascara tablets, elixir, dry exract, liquid extract. 4- Rhubarb powdered, tincture. 5- Danthrone 6- Frangula bark 39 - Flavonoidal compounds are considered as the largest group of naturally occurring phenols. - Flavonoidals constitute the majority of the yellow colored plant pigments. - Many flavonoidal compounds present as a glycosidic or as a free forms. - All derived from the same parent nucleus, 2-phenylbenzopyran (flavan), thus they have a basic C-15 skeleton. Flavonoidal compounds are classified according to the oxidation level of central pyran ring they are classified into flavones, isoflavones, flavonols, flavanones, (true flavanoids) anthocyanidins, chalcones and aurones. True flavones, are 2-phenyl chromones (2-phenyl benzopyrone), while isoflavones are 3-phenyl chromones der. Flavonols are 3-hydroxyflavones, while flavanones are 2,3dihydro der. of flavones (2,3-double bond is lacking). 40 (2-phenylbenzopyran) 8 9 7 3' 2' 1 O (2-phenylbenzopyrone) O 1' 4' 2 6 5 10 4 3 6' 5' Flavan O Flavone O O OH O O Flavonol Isoflavone O H H O Flavanone Anthocyanidines, chalcones and aurones are lack the typical flavone structure. Anthocyanidins and its glycosides (anthecyanins) are ionic oxonium salts. This is responsible for the permanent blue, purple, violet, mauve, and red color of flower, fruits and leaves of higher plants. Anthocyanidins and anthecyanins are soluble in polar solvents. 41 Cyanidin chloride is an example of anthocyanidines . 8 9 2' + O 3' R 4' OH 6 5 3 10 8 OH 1' 7 6' X 5' R - 9 Cl + O 2' OH 1' 7 4' OH 6 5 Anthocyanidins 3' 10 3 OH 6' 5' Cyanidin chloride OH Chalcones, have no central pyrone ring, so they are not true flavonoidal compounds. The parent compound chalcone, is chemically phenyl-styryl ketone, or benzylidene acetophenone. Aurones are oxidized forms that are obtained by enzymatic oxidation. Instead of the central pyrone ring of the normal flavonoidal structure, aurones have five membered ring. O CH O Chalcon O Aurone 42 Flavonoids dissolve in alkalis give intense yellow color solution, on the addition of acid become colorless. Flavonoids exhibit strong fluorescence under UV light. Flavonoidal glycosides are soluble in water and alcohol. Ethylacetate is the solvent of choice for the extraction of flavonoids from aqueous solution. Flavonoids compounds may be characterized through the investigation of their UV Spectra, that usually show two main bands, 1- Band at higher wavelength (band I) which is attributed to the cinnamoyl fraction of the flavonoidal structure Why?. 2- Band at lower wavelength (band II) which is due to the benzoyl fraction of the flavonoidal structure. 43 Benzoyl O B A R Cinnamoyl O A I Band II Band 200 Wave length Hypsochromic shift 400 Bathochromic shift Band I >> 300 nm If R= H R=OH R=O-substitution Flavones flavonols 3-sub flavonol Band I: 304-350 nm Band I: 352-385 Band I: 328-357 Band II << 300nm (250-280 nm) Note: More OH in ring A: Bathochromic shift in band II. More OH in ring B: Bathochromic shift in band I. Shift reagents: Back to lab. 44 1- Diosmin: flavone glycoside Occurance: buchu leaves, Barosma crenulata F. Rutaceae. Uses: diuretic and diaphoretic action of the leaves is owed in part to diosmin, and in part to diosphenol, the main constituent of the volatile oil of the leaf. OH Rha-Gl O O B A OCH3 OH O Diosmin Upon hydrolysis, diosmin yields rhamnose, glucose and diosmetin. 2- Rutin and quercetrin: are examples of flavonol glycosides a- Rutin occurs in the leaves of buckwheat. It is the 3rhamnoglucoside (called rutinose) of the genin quercitin. It gives on hydrolysis the aglycone (quercitin) beside one molecule of glucose, and one molecule of rhamnose. 45 Rutin is used to 1- Decrease capillary fragility. 2- It is a biflavonoids that plays a true vitamin function. b- Quercitrin is quercitin 3-O-rhamnoside. It occurs in the bark of Quercus tinctoria. Quercitrin yield upon acid hydrolysis rhamnose and quercetin. The aglycone quercetin occurs in bearberry leaves (Uva Ursi) and has a diuretic action of the leaves. OH O HO B A OH OR OH O Quercetin: R=H Quercetrin: R= rhamnosyl Rutin: R=rutinosyl 3- Hesperidin: it is an example of flavanones. It is the main flavonoidal glycoside of citrus fruits. 46 OH R O O B A OCH3 OH O Hesperitin R:H Hesperidin R:rutinosyl Upon hydrolysis by acid, hesperidin gives rhamnose, glucose and hesperitin. Uses: 1- Hesperidin appears to be identical to vitamin P (citrin). 2- It is necessary for absorption and retention of vit C that lead to decrease capillary fragility. 3- Decrease CVD and HTN. Uses of flavonoids: 1- Increase capillary resistance and decrease vitamins C & P deficiency. 2- They are recommended in the treatment of thrombopenia (blood coagulation). 3- They are reported of value in the treatment of influenza, when given with ascorbic acid. 47 Isoflavone: 1- Genistein show significant oestrogenic activity. O HO OH O OH 2- Rotenoids employed as insecticide. O O O O Flavono-lignans Coupling of a flavonoid moiety with hemi-lignan molecule by oxidative coupling. 48 O OH B A OR OH + OH OH O Hemi-lignan moiety Flavonoid moiety O OH B A O OH O OH O OCH3 OH Flavonolignan The leaves and fruits of Silybum marianum family Compositae contain silymarin (silybin). O OH B A OH OH O O O OH OCH3 Silybin OH 49 1- Silymarin is a very effective lipotropic and hepato protective therapy. 2- It is a free radical scavenger. 3- Supportive treatment of acute and chronic alcoholic poisoning and toxin induce hepatitis. 4- It is used for treatment of liver cirrhosis caused by plant toxins (mushroom, amanita), silymarin is applied as intravenous injection. 5- Silymarin is available in the market in the form of tablets, effervescent granules. Trade name legalon, silyhexal, silirex…etc. Synthetic flavonoids Flavoxate: O O N O B A CH3 O Flavoxate Uses: To remove pain (anti-spasmodic) and anti-inflammatory of the genitor urinary tract. Flavoxate tablets are available under several names: Urispas, Uronid, Spasurit, Genurin). 50 * Saponins are a group of amorphous colloidal glycodides which is wiedly distributed in the higher plants. * Have ability to form lasting foam when shaking in aqueous solution. * They are excellent emulsifying agents (modify surface tension). * Formerly used as detergents to replace soap (e.g., quillaia). * Saponins are colorless and optical active. They form colloidal solution with water and are soluble in alcohol and dilute alcohols. * Saponins have haemolytic properties, they precipitate the cholesterol and lethisins that exist in the memberanes of the red blood cells and thus haemoglobin is liberated. So, saponins are extremely toxic when injected into the blood stream. However, they are not harmful when taken orally. * Saponins are difficult to purify. However, they precipitated from solutions containing them by the addition of a solution of the sterol, filtering off the insoluble sterolsaponin compound and boiling it with toluene which resolves the compound again into sterol (which is soluble in toluene) and saponin (which is insoluble in toluene). 51 Chemically: Saponins are classified according to the genin part into: 1- Steroidal type C25. 2- Triterpinoidal type C30. Both types of saponins have the glycosidic linkage at position 3. O O COOH R2 HO HO R1 Diosgenin Quillaic acid: R1=CHO, R=OH Olianolic acid R1=CH3, R2=H Medicinal importance of saponins: 1- The steroidal saponins are structurally related to modern synthetic compounds that have a therapeutic significance, such as adrenocortecoids and the sex hormones. So, they are a suitable precursors in the partial synthesis of these hormones, e.g., Diosgenin (sapogenins) isolated from the rhizome of Dioscoria species. 52 CH3 OH O CO OH O O O Testosterone Progesterone O CH2 OH O O CO OH O HO Cortisone Diosgenin 2- Saponins increase the rate of absorption of many pharmacological active substances (e.g., cardiac glycosides). 3- Many saponin-containing drugs are used as expectorants (e.g., Ipeca, Senaga and liquorice) as their contents of saponins stimulate bronchial secretion and also activate the ciliary epithelium of the bronchi. a-The triterpenoidal saponin glycoside, glycyrrhizin, is the main sweet principle of liquorice. It is calcium and potassium salts of glycyrrhizic acid, which in tern is the diglucuronic acid glycoside of glycyrrhitinic acid. 53 COOH O Glucuronic-glucuronic O B-Glycyrrhitinic Glycyrrhizic acid Glycyrrhizin =Ca, K b- Beside being a valuable flavouring and sweetening agent, liquorice has demulcent, expectorant and antispasmodic action. All these activities attributed to the saponin, glycyrrhizin. c- Recently, glycyrrhizin was shown to be effectively in gastric ulcer treatment and have a cortisone like action in rheumatic arthritis and other inflammatory diseases. Saponins drugs officially in the B.P and U.S.P: 1- Quillaia bark: used as emulsifier. 2- Liquorice root: used as flavouring agent and expectorant. 54 1- Tannins are widely distributed phenolic plant constituents. It is characterized by being able to combine with proteins of animal hides thus preventing their putrefaction and converting them into leather (true tannins). 2- Tannins are detected qualitatively by Goldbeater’s skin test (a tanning test), and can be quantitatively estimated by absorption on standard hide powder. Only high molecular weight tannins that are capable of tanning hide. It is more acceptable to define true tannins as those high molecular weight phenolic plant constituents that can be detected by Glodbeater’s skin tanning test. 3- True tannin solutions have the ability of precipitating soluble proteins (gelatine), heavy metals, alkaloids and glycosides. 4- This will exclude simple molecular weight compounds such as gallic acid, catechin, flavan-3,4-diol and chlorogenic acid, that usually coexist with true tannins. 55 These simpler tannins like compounds are referred to as pseudotannins. HO HO OH HO OH HO COOC6 H11 O5 COOH Glucogallin Gallic acid OH CH=CH-COO HO OH HO HO O OH Chlorogenic acid OH OH Flavan-3,4-diol Hydrolysable tannins Condensed tannins OH 56 1- Hydrolysable tannins: a- These can be hydrolyzed by acids or enzymes to give phenolic acids (gallic or ellagic) and glucose, so called phenolic acid glycosides. b- Tannins of gallic acid are called gallitannins and those of ellagic acid is called ellagitannins. c- Dry distillation of hydrolysable tannins gives pyrogallol. This class is named pyrogallol tannins. d- Gallitannins and ellagitannins react with ferric salts to give bluish color precipitate. 2- Condensed tannins: a- These are more resistant to hydrolysis upon prolonged heating with acids. b- They undergo decomposition (not hydrolysis) to give a red soluble compound (phlobaphane). c- Condensed tannins are derived from catechin and flavan, 3,4-diol. d- Dry distillation of condensed tannins gives catechol. This class is named catechol tannins. e- Being phenolic, it reacts with ferric salts to give greenish color precipitate. 57 1- Salicin: Salicin is classified as: 1- Alcoholic glycoside, as it contains free primary alcoholic group. 2- A phenolic glycoside, as its aglycone is phenolic in nature. CH2 OH Gl O Salicin 1- Salicin is obtained from different species of Salix, the principle commercial source is Salix fragilis. 2- Salicin is used for many years as a remedy in the treatment of fever and rheumatism. 3- It is now used as an analgesic-antipyretic in case of periodic fever. It is better tolerated in the stomach than sodium salicylate, asprin and other antipyretics and antiinflammatory agents, which have largely displaced in medical practice. 58 4- Salicin is hydrolyzed by the enzyme emulsin into saligenin (Salicyl alcohol) and glucose. 5- Acid hydrolysis of salicin gives glucose and a phenolic ether called saliretin which is a condensation product of two molecules of saligenin. CH2 OH Gl O Acid Enzyme CH2 OH CH2 OH HO + Glucose Saligenin O + Glucose CH2 OH Saliretin 6- Oxidation of saligenin gives salicylic acid and this accounts for the medicinal value of salicin. 1- Arbutin is a phenolic glycoside that occurs in bearberry leaves Arectostaphyllos uva ursi. 2- When hydrolysed with acids or with emulsin it yields glucose and hydroquinone. 59 3- It is used as diuretic and also has bactericidal action. This activity is due to the hydroquinone given by hydrolysis. 3- Uva ursi leaf contains also methylarbutin (the methyl ether of arbutin), that also contributes to the diuretic and urinary antiseptic action of the leave. OH OCH3 O-Gl O-Gl Arbutin Methylarbutin 1- Glucovanillin is a glycosidal constituent of green vanilla pods. 2- The fruits of the plant (pods) are collected and carefully cured. To permit enzymatic action on the glycoside with the liberation of vanillin (the aglycone) which is the principal flavouring constituent of the pods. 60 3- Vanillin is widely used as a flavouring agent. It may be obtained from vanilla pod or prepared from the glycoside coniferin, lignin or from the phenolic volatile oil constituents eugenol. CHO CHO OCH3 OCH3 O-Gl OH Glucovanillin Vanillin 1- From Coniferin and lignin CH=CH-CH2 OH CH=CH-CH2 OH Hydrolysis CHO Oxidation OCH3 OCH3 OCH3 OH O-Gl Coniferin OH Coniferyl alcohol Vanillin 2- From Eugenol CH2 -CH=CH2 CH=CH-CH3 KOH Oxidation OCH3 OH Eugenol CHO OCH3 OH isoeugenol OCH3 OH Vanillin 61 The bulk of vanillin which is produced commercially is prepared from lignin, which gives upon hydrolysis coniferyl alcohol. Hydrolysis Lignin coniferyl alcohol Lignin is obtained in extremely large amounts as a by product of timber industry. 1- These are glycosides that are yield hydrocyanic acid as one of their hydrolytic products. 2- Plant containing these glycosides are toxic. 3- The aglycone part is cyanohydrin of a carbonyl compound (condensation product of HCN with an aldehyde or keton). 4- The majority of cyanogenic glycosides are derived of benzaldehyde cyanohydrin. O OH HCN Sugars C CH H CN Benzaldehyde Mandilonitrile CH3 C CH3 Acetone Mandilonitrile glycosides O HCN CH3 OH C CH3 O-Gl CH3 Sugars CN Acetone cyanohydrin C CH3 CN Linamarin 62 D-Mandelonitrile gentiobioside 1- Amygdalin is the most widely distributed cyanophore glycoside. 2- It occurs in several Prunus species, and is obtained from bitter almonds (Prunus amygdalus Var. amara Family Rosaceae). 3- Amygdalin is considered as gentiobioside of Dmandelonitrile. Gentiobioside is a reducing disaccharide consisting of two molecules of β-glucose linked by β-1,6 linkage. CN C O O 6 CH2 OH H O 5 H O 5 2 3 2 3 1 6 CH2 1 4 4 Amygdalin CN C O 1 6 CH2 OH 5 3 4 H O 2 Prunasin 63 4- Acid hydrolysis of amygdalin split two molecules of glucose and one molecule of mandelonitrile. The latter decomposes spontaneously to form benzaldehyde and HCN. 5- Different enzymes act upon amygdalin in different ways: Amygdalase Gl-Gl-O C CN Prunase Glocose + Prunasin Prunase Glucose + HCN +Benzaldehyde Gentiobiose + Benzaldehyde + HCN H Amygdalin Emulsin or acid Glucose + Benzaldehyde + HCN The plant material is cutted into small fragments and then a filter paper moistened with sodium picrate is then suspended in the neck of the flask, the flask is stoppered and incubated in a warm place (40˚C) for about 30-60 min. By this time, the coexisting enzymes act upon the glycosides with the liberation of HCN which turns, the sodium picrate paper convert to brick red color. 64 Thioglycosides 1- A number of plants of the family Cruciferae yield glycosides containing sulphur. 2- Hydrolysis of these, yield volatile genins of thiocyanate structure e.g., mustard oils. 3- The best known compounds Sinigrin and Sinalbin, two glycosides occurring in black mustard and white mustard seed respectively. 4- The glycosides and their specific enzymes are found in different cell in the seeds. They donot interact until they are brought together by the distruction of the cell walls. 5- The general structure of thioglycosides is: S-GL X R C + N-OSO3 6- The anion is called the glucosinolate ion, R may be aliphatic or aromatic. The cation (X) may be a simple metal ion or a complex organic cation, e.g., sinapine ion of sinalbin. 65 S-GL S-GL CH2 HO CH-CH2 -C CH2 C N-OSO3 - Sinapine+ N-OSO3 K Sinigrin 6- Sinigrin Sinalbin gives allylisothiocyanate upon (volatile hydrolysis, oil of glucose, mustard) and potassium acid sulphate. 7- Hydrolysis of the glycoside sinalbin gives a phenolic isothiocyanate (Acrinyl isothiocyanate), glucose and the acid sulphate of a quaternary alkaloid, sinapine. + CH3 O HO CH-CH-COO-CH2 -CH2 -N CH3 CH3 CH3 CH3 O Sinalpine cation 8- Black and white mustard seeds are used as rubefacients and counter irritants. These effects are attributed to their contents of thioglycosides. 66 Aglycone 1- coumarin (benzo-α-pyrane). 2-coumarin derivative (hydroxyl and methoxy coumarins). 3- Umbelliferone [7-hydroxy coumarin] is the lactone of umbellic acid which occurs both in the free state and in the form of glycosides in some resins of the Umbelliferae (Asafetida and galbanum). O O O a-pyrone O HO coumarin O O umbelliferone 4- Coumarin and its derivatives give blue or violet fluorescence in aqueous ammonical solutions (conjugated double bond system). This is made use of in qualitative testing for coumarin, coumarin derivatives and coumarin glycosides and drugs containing them. 5- The oleo gum resin galbanum that contains umbelliferone in a free state is distinguished from asafoetida that contains only combind umbelliferone, by the addition of ammonia to its aqueous alcoholic extract, when the characteristic blue fluorescence is given. Asafetida responds positive to the fluorescence test only after acid hydrolysis.