11.6B Be Specific - Texarkana Independent School District
advertisement

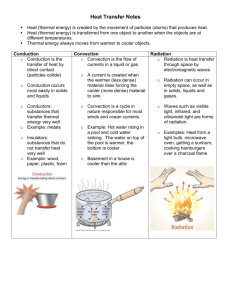
Focus Plan Texarkana Independent School District GRADING PERIOD: WRITER: IPC + Chemistry – 5th six weeks, PLAN CODE: L. Petty COURSE/SUBJECT: 11th grade science GRADE(S): 11th TIME ALLOTTED FOR INSTRUCTION: 1-1½ hours TITLE: Be Specific LESSON TOPIC: Specific Heat TAKS OBJECTIVE: Objective 5 The student will demonstrate an understanding of motion, forces, and energy. FOCUS TEKS AND STUDENT EXPECTATION: 11.6 The student knows the impact of energy transformations in everyday life. The student is expected to: (B) investigate and demonstrate the movement of heat through solids, liquids, and gases by convection, conduction, and radiation. Objective 1: The student will demonstrate an understanding of the nature of science. 11.1 The student, for at least 40% of instructional time, conducts field and laboratory investigations using safe, environmentally appropriate, and ethical practices. The student is expected to: (A) demonstrate safe practices during field and laboratory investigations 11.2 The student uses scientific methods during field and laboratory investigations. The student is expected to: (A) plan and implement investigative procedures including asking questions, formulating testable hypotheses, and selecting equipment and technology (B) collect data and make measurements with precision (C) organize, analyze, evaluate, make inferences, and predict trends from data (D) communicate valid conclusions SUPPORTING TEKS AND STUDENT EXPECTATIONS: CONCEPTS Energy Heat Amount Specific Heat Value ENDURING UNDERSTANDINGS/GENERALIZATIONS/PRINCIPLES The student will understand that Energy exists in many forms in the world around us. Heat is thermal energy that flows from something that is hotter to something that is cooler. The amount of heat absorbed changes the temperature of an object. The temperature change of a substance depends on the nature of the substance as well as the amount of applied heat. A higher specific heat means that more energy will need to be applied to get the object’s temperature to increase. This means it will stay cool longer. I. SEQUENCE OF ACTIVITIES (INSTRUCTIONAL STRATEGIES) A. Focus/connections/anticipatory set When students come in to class, have a pan (not a copper-bottomed one) on a hot plate and act like you are cooking something in the pan. After a little while, act impatient and say that it will never heat up at this rate. Pull out a copper-bottom pan and put that on the hot plate instead. B. Instructional activities (demonstrations, lectures, examples, hands-on experiences, role play, active learning experience, art, music, modeling, discussion, reading, listening, viewing, etc.) 1. Discussion Explain to students that some metals heat up faster than others. This is a physical property of substances called specific heat. Specific heat is, technically, the amount of heat that is needed to raise the temperature of 1 kg of that substance by 1oC. If a substance heats up quickly, it has a low specific heat. C. Guided activity or strategy Use Transparency Master – Specific Heat Calculations to show students how to complete their calculations. D. Accommodations/modifications Students requiring accommodations may need help with practice calculations. E. Enrichment Students requiring enrichment may be given additional calculations to do and may serve as peer tutors. II. STUDENT PERFORMANCE A. Description Complete Lab – Be Specific. B. Accommodations/modifications Students requiring accommodations should be assigned a peer tutor, especially for the math calculations. C. Enrichment Students requiring enrichment should serve as peer tutors. III. ASSESSMENT OF ACTIVITIES A. Description Grade Lab Worksheet – Be Specific. B. Rubrics/grading criteria Questions should be graded at 4 points each. Each blank in the first data table should be graded at 1 point each. For the temperature recordings in the cup (Table 2), grade the recordings for each substance at 5 points per substance (basically, if they recorded temperatures, they will get these points). C. Accommodations/modifications Students requiring accommodations may need to work calculation questions with a peer tutor or may need to be given some leeway in grading for these questions. D. Enrichment Students requiring enrichment may be assigned additional samples to test or may be given the assignment to graph their data. E. Sample discussion questions 1. 2. 3. 4. IV. Which substance had the most mass? They were all the same. Why was the mass kept constant? That was a constant, to make sure that the specific heat of the substance was the only thing affecting the temperature difference. Copper is widely used in electrical wiring for houses because it allows electricity to pass through it easily. Do you think there might be a relationship between the thermal and electrical conductivity of substances. It is possible because copper is both a good thermal and good electrical conductor. Why did you have to stir the water in the Styrofoam cup while taking the temperature? Water is not a good thermal conductor. TAKS PREPARATION A. Transition to TAKS context 1. A teacher provides an unlabeled drawing of a beaker of water being heated over the flame of a gas burner. He asks his students to supply the missing captions in order to illustrate how convection currents move heat through water. What captions will enable the drawing to illustrate how convection currents move heat through water? (a) y: cool, more dense; z: warm, less dense (b) y: warm, more dense; z: cool, less dense (c) y: cool, less dense; z: warm, more dense (d) y: warm, less dense; z: cool, less dance 2. Heat moves through most liquids and gases by actually moving the material. What is this type of heat movement? (a) radiation (b) infrared (c) convection (d) conduction B. Sample TAKS questions Spring 2003 1. Heat convection occurs in gases and liquids. Heat convection does not occur in solids because solids are unable to ____. (a) absorb heat by vibrating (b) transfer heat by fluid motion (c) emit radiation by reflecting light (d) exchange heat by direct contact 2. A solar heater uses energy from the sun to heat water. The heater’s panel is painted black to ___. (a) improve emission of infrared radiation (b) reduce the heat loss by convection currents (c) improve absorption of infrared radiation (d) reduce the heater’s conducting properties Spring 2004 3. The moon’s surface becomes hot during the long lunar day because the sun transfers heat to the moon. This heat transfer is accomplished almost entirely through the process of _____. (a) convection (b) refraction (c) conduction (d) radiation 4. In which container is the substance unable to transfer heat by convection? V. KEY VOCABULARY conduction mass VI. RESOURCES specific heat capacity volume A. Textbook – none needed B. Supplementary materials/equipment Transparency Master – Specific Heat Calculations Instructor’s Copy – Specific Heat Calculations Lab Instructions – Be Specific Lab Worksheet – Be Specific Instructor’s Copy – Be Specific C. VII. Technology FOLLOW UP ACTIVITIES (reteaching, cross-curricular support, technology activities, next lesson in sequence, etc.) A. Reteach Go over graded lab papers. B. Next lesson in sequence All subjects – convection and/or radiation. VIII. TEACHER NOTES Before lab: 1. Make a class set of Lab Instructions – Be Specific 2. Make enough copies of Lab Worksheet – Be Specific for each student to have one. 3. If using new specific heat sets, or ones without standards given, run the lab before giving it to your class to double check the standard values and to calculate normal percent error. 4. Assemble the materials since this may require purchasing styrofoam cups. During lab: 5. To make for equitable sharing of specific heat sets, it might be best to assign which 4 substances each group will be testing. 6. Make sure the beakers of boiling water to no go dry. The water should fully cover all metal cylinders in each beaker.