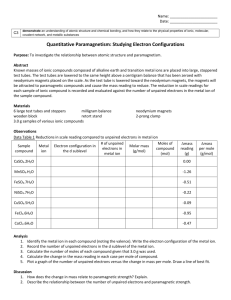
Cr2(SO4)3∙n H2O
advertisement

Activity 1: Coordination Chemistry: Metal Salts, Metal Complexes and their Names Introduction Given the formula for a common inorganic substance, you will be at a disadvantage if you cannot envision what is possibly in the substance and the name of the substance. This activity gets us started on our study of coordination chemistry. It also includes a brief review of the periodic table. Learning Objectives Review: sketch from memory (and prior experience or this activity) the relative positions of the following in the periodic table: metals and nonmetals; semimetals; alkali and alkaline earth metals; transition metals; lanthanide metals (rare earth metals) and actinide metals. Given a formula of a metal-containing common salt, be able to name the metal from the symbol, state what ions (and their names) are in the compound, and visualize the possible entities in the crystals depending on what the coordination number of the metal ion is and on what ions or molecules in the formula could be positioned in the coordination sphere of the metal ion. Write names for the complex salts of metal ions. Criteria for Success Thoroughness and correctness in focusing on your problem and answering the questions below. Quality of group interaction and participation of all persons in accomplishing the learning objectives. How well the group interacts with the instructor as a resource. Quality and correctness of group sharing with the class about your assigned problem. Information and Resources Chapter 2 in Rodgers (Glen E. Rodgers, “Descriptive Inorganic, coordination and Solid-State Chemistry,” Brooks/Cole, Thompson Learning, USA, 2002, ISBN 0-12-592060-1). Normally, you will be given a reading assignment and a homework problem or two to prepare for the activity. Today we will use the text book and instructor as resources to initiate our study. See especially pages 18 to the end of the chapter 2. Mini-lecture on coordination chemistry, metal complexes, and on nomenclature. Normally, the activity will be introduced with a mini-lecture of no more than ten minutes followed by questions and discussion. This does not substitute for your pre-activity preparation by reading and working the assigned problem. If you have something unclear from the reading, bring it to class. Now is the time to ask. Rodgers Table 2.3 for naming of ligands and Rodgers Table 2.4 for naming simple compounds. Examples 1 – 8 following the Tables. Review of rules for naming compounds: 1. All cations precede anions both in name and formula. 2. Cations are listed in alphabetical order except for the acidic hydrogen which is usually handled in the name of the anion (example hydrogenphosphate). 3. Anions with regular endings are cited in alphabetical order. Regular anion endings: -ide, -ite, ate. Activity for Fundamental Inorganic Chemistry contributed by Susan C. Jackels, Seattle University. 4. Anions that appear more than once (have subscript number) are indicated by using the multiplicative prefixes: bis (two), tris (three), tetrakis (four), penta (five), hexa (six), hepta (seven), octa (eight), nona (nine), deca (ten) and so on. 5. Water of hydration is recognized by its position at the end of the formula and preceded by a dot. The name includes waters of hydration at the end showing its numerical prefix as in 4 above followed by hydrate (ex. hexahydrate for ∙6H2O). Note that often some or all of these waters of hydration are coordinated to the metal ion. Sometimes “anhydrous” is put before the name to emphasize that no water of hydration is present. Plan (This is a typical plan – in this activity there will be one problem per group, in other activities there may be one problem per individual but you will still help each other in groups and we will discuss the activity using group contributions.) 1. Form groups of three. Choose roles of captain, recorder and reflector. Roles: Captain -- leads discussion, keeps the group on track toward accomplishing plan and learning objectives. Recorder -- writes responses for critical thinking questions on the group report sheet, and records the results of the assessment discussion. Reflector – encourages the group to think more deeply and improve quality of responses and leads the assessment discussion after the group has completed the activity. 2. Answer the critical thinking questions listed below. While the group supports each person, you will be individually responsible for recording the responses and presenting the discussion of your assigned problem (if individually assigned). 3. The instructor will collect the individual or group contributions into a class report. 4. Refer back to the criteria for performance success and assess your group’s work. 5. Turn in the group report and the assessment report. 6. Be ready to participate in the class discussion of each problem. Assignment I. Naming Metal Salts Your group will be given a vial with a sample of a metal salt in it. The vial is labeled with the formula of the salt as it appeared on the bottle. Observe the salt but do not open the bottle (toxic). The metal salts for the class are: Cr2(SO4)3∙n H2O MnCl2∙4H2O Fe(NH4)2(SO4)2∙6H2O CoCl2∙6H2O NiSO4∙6H2O CuCl4(NH4)2∙2H2O Activity for Fundamental Inorganic Chemistry contributed by Susan C. Jackels, Seattle University. ZnCl2 Critical Thinking Questions Fill out the following report for your sample. Names of group members: ____________________________________________ Formula of compound as seen on vial: _______________________________________ Appearance of compound: ________________________________________________ 1. From the formula for the compound, what are the anions and cations in the formula? Include charges and proper names. 2. Are there any neutral molecular entities in the compound? What are they and how many are there in each formula unit? 3. Write a name for the compound as it is shown in the formula. Example: NaCl would be sodium chloride, FeCl2 would be anhydrous ferrous chloride, CoCl3∙5H2O would be cobaltic chloride pentahydrate or cobalt(III) chloride pentahydrate. 4. The formula for the compound on the commercial bottle did not specify what was in the coordination sphere of the metal ion. What entities in the formula can possibly be coordinated to the metal ion? 5. Assuming the coordination number of the metal is six, write some possible formulas for metal complexes that could be in the salt. For the example in 3 above, CoCl3∙5H2O could have [Co(H2O)5Cl] 2+ and two Cl- in the crystal. Once you have attempted this, consult with the instructor to find out what the likely possibilities are based on the chemistry of the metal ion in your salt. 6. Assuming the coordination number of the metal is four, write some possible formulas for metal complexes that could be in the salt. Again, consult with the instructor. 7. For two or more of the responses in questions 5 and 6, write the formula for the salt, including the complex ion in brackets and write the name for the compound. For the example in 3 and 5 Activity for Fundamental Inorganic Chemistry contributed by Susan C. Jackels, Seattle University. above, the salt would be [Co(H2O)5Cl]Cl2 and the name would be pentaaquachlorochromium(III) chloride. 8. Be ready to present about your salt to the class. Turn in your group report that will be gathered with other groups into a class report to be posted on the class web site. Post-activity assignment: II. More practice naming coordination complexes and coordination compounds (see tables 2.3 and 2.4 and rules on page 22) 1. Write the name for [Pt(NH3)4Cl2] Cl2 2. For the compound in question 1, what is the oxidation state (charge) on Pt? What are the ligands? What is the complex ion? And what is/are the counter ion(s)? 3. Write the name for [CuCl4]2-. What is the coordination number of this complex ion? 4. Write the structural formula for chlorophosphine palladium(II)-μ-dichlorochlorophosphine palladium(II). What does the μ mean? 5. Write the formula for the complex ion hexaaquamanganese(II). 6. Write the formula for tris-bipyridineruthenium(II) chloride. Activity for Fundamental Inorganic Chemistry contributed by Susan C. Jackels, Seattle University.