Top of Form 1. Where is metamorphic rock frequently found? on
advertisement

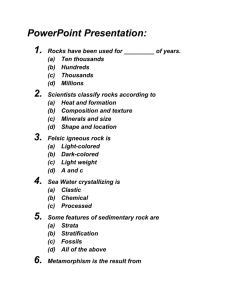
1. Where is metamorphic rock frequently found? 1. 2. 3. 4. on mountaintops that have horizontal layers containing marine fossils within large lava flows as a thin surface layer covering huge areas of the continents along the interface between igneous intrusions and sedimentary rocks -------------- 2. The recrystallization of unmelted material under high temperature and pressure results in 1. metamorphic rock 3. igneous rock 2. sedimentary rock 4. volcanic rock -------------- 3. The diagram represents five rock samples. Figure 1 If granite were subjected to intense heat and pressure, it would most likely change to 1. conglomerate 3. gneiss 2. sandstone 4. basalt -------------- 4. 6. The diagrams represent cross sections of four rock samples. Each cross section illustrates the sediments, minerals, or structural appearance of the rock samples. The diagram represents a rock with a distorted layer structure. The distorted structure of this rock is most likely the result of Figure 2 Which rock sample is most likely a nonsedimentary rock? 1. A 2. B 3. C -------------5. The diagram represents a geologic cross- 1. 2. 3. 4. a long period of weathering glacial activity wind erosion extreme pressure -------------- section. Figure 3 At which location would quartzite most likely be found? 1. A 3. E 2. B 4. D -------------- 7. The diagram shows the structure of a student-developed chart for identifying some rock samples. The circles labeled choice 1 through choice 4 represent decision-making steps leading either to path (a) or path (b). Choice 5 has not been completed. Figure 4 Which rock specimen should lead the student to choice 4, path (a)? 1. peridotite 3. gneiss 2. quartzite 4. dolostone -------------- 8. Which rock sample is most likely a foliated metamorphic rock? 1. 3. 2. 4. -------------- 9. Which rock is foliated, shows mineral alignment but not banding, and contains medium-sized grains of quartz and pyroxene? 1. phyllite 3. gneiss 2. schist 4. quartzite -------------- 10. Base your answer on the geologic cross section of bedrock. A through G identify rock layers and Q represents a fault. Lines W, X, Y, and Z are locations of unconformities. The rocks have not been overturned. Figure 5 Which rock most likely formed in the zone of contact between rock E and rock F? 1. obsidian 3. metaconglomerate 2. slate 4. sandstone -------------- 11. Which two kinds of adjoining bedrock would most likely have a zone of contact metamorphism between them? 1. shale and conglomerate 3. limestone and sandstone 2. shale and sandstone 4. limestone and granite -------------- 12. Base your answer to the question on the geologic cross section below. Location A is within the metamorphic rock. Figure 6 The metamorphic rock at location A is most likely 1. marble 3. phyllite 2. quartzite 4. slate -------------- 13. Which property is most useful in mineral identification? 1. hardness 3. size 2. color 4. texture -------------- 14. The mineral mica breaks evenly along flat sheets mainly because of its 1. 2. 3. 4. atomic arrangement chemical composition hardness density -------------- 15. The table shows the physical properties of nine minerals. Figure 7 Which mineral has a different color in its powdered form than in its original form? 1. pyrite 3. kaolinite 2. graphite 4. magnetite -------------- 16. The relative hardness of a mineral can best be tested by 1. scratching the mineral across a glass plate 2. squeezing the mineral with calibrated pliers 3. determining the density of the mineral 4. breaking the mineral with a hammer -------------17. The data table shows the composition of six common rock-forming minerals. The data table provides evidence that 1. 2. 3. 4. the same elements are found in all minerals a few elements are found in many minerals all elements are found in only a few minerals all elements are found in all minerals -------------- 18. 23. Certain minerals usually break along flat surfaces, while other minerals break unevenly. This characteristic is due to the Different arrangements of tetrahedra in the silicate group of minerals result in differences in the minerals' 1. luster of the mineral 2. age of the mineral 3. internal arrangement of the mineral’s atoms 4. force with which the mineral is broken 1. age, density, and smoothness 2. cleavage, color, and abundance 3. hardness, cleavage, and crystal shape 4. chemical composition, size, and -------------- origin 19. -------------- Which is the best explanation for the cleavage of mica into thin layers? 1. 2. 3. 4. the high density of mica the arrangement of the atoms in mica the softness of mica the impurities found in mica -------------- 20. Quartz mineral samples are best identified by their 1. hardness 3. size 2. color 4. mass -------------- 21. 24. Which common mineral fizzes when dilute hydrochloric acid (HCl) is placed on it? 1. calcite 3. quartz 2. feldspar 4. talc -------------- 25. Which of the following minerals has metallic luster, silver color, black streak, and contains iron? 1. Graphite 3. Magnetite 2. Galena 4. Pyrite -------------- 26. The diagrams represent fractured samples of four minerals. Which of the following statements is not true about the mineral hematite? 1. Hematite can appear metallic or earthy. 2. Hematite has a composition that contains iron and sulfur. 3. Hematite has a red-brown streak. 4. Hematite is an ore of iron. -------------- Which mineral property is best illustrated by the samples? 1. hardness 3. cleavage 2. streak 4. density -------------22. Which property of a mineral most directly results from the internal arrangement of its atoms? 1. volume 3. crystal shape 2. color 4. streak -------------- 27. Base your answer on the diagram, which shows three minerals with three different physical tests, A, B, and C, being performed on them. Figure 8 Which sequence correctly matches each test, A, B, and C, with the mineral property tested? 1. 2. 3. 4. A–cleavage; B–streak; C–hardness A–cleavage; B–hardness; C–streak A–streak; B–cleavage; C–hardness A–streak; B–hardness; C–cleavage -------------- 28. The photograph below shows a broken piece of the mineral calcite. The calcite breaks in smooth, flat surfaces because calcite 1. 2. 3. 4. is very dense is very soft contains certain impurities has a regular arrangement of atoms -------------- 29. 34. Which element is most abundant in Earth’s lithosphere? [Refer to figure 7 in question 15] 1. oxygen 3. hydrogen 2. silicon 4. nitrogen Which mineral is commonly found in granite? -------------30. The diagrams represent four rock samples. Which rock took the longest time to solidify from magma deep within the 1. quartz 3. magnetite 2. olivine 4. granite -------------35. Earth? The size of the mineral crystals found in an igneous rock is directly related to the 1. 1. density of the minerals 2. color of the minerals 3. cooling time of the molten rock 4. amount of sediments cemented together 3. -------------36. 2. 4. -------------- [Refer to figure 1 in question 3] 31. Which sample is igneous and has a coarse texture? A fine-grained igneous rock contains 11% plagioclase, 72% pyroxene, 15% olivine, and 2% amphibole. This rock would be classified as 1 sandstone . 3 basalt . 2 conglomerat . e 4 granit . e 1. granite 3. gabbro 2. rhyolite 4. basalt -------------37. -------------32. Which statement best describes the properties of basalt? Rhyolite is an example of a 1. 2. 3. 4. 1. fine-grained and mafic 2. fine-grained and felsic 3. coarse-grained and mafic 4. coarse-grained and felsic monomineralic igneous rock polymineralic igneous rock monomineralic sedimentary rock polymineralic sedimentary rock -------------- -------------- 33. 38. The best evidence for determining the cooling rate of an Large crystal grains in an igneous rock during its solidification is provided by 1. 2. 3. 4. index fossils faults in the rock the crystal size of its minerals the disintegration of radioactive substances -------------- igneous rock are an indication that the crystals formed 1. over a long period of time 2. under low pressure 3. near the surface of the Earth 4. at a low temperature -------------- 39. The diagram represents a scheme for classifying rocks. The letter A, B, C and X, Y, Z represent missing labels. Figure 9 Which processes would form the type of rock that is represented by circle B? 1. 2. 3. 4. deposition and compaction weathering and erosion melting and solidification faulting and folding -------------- 40. The four igneous rocks below are classified into two groups: What is the basis for this classification? 1. 2. 3. 4. density color crystal grain size mineral content -------------- 41. The diagrams represent five different rock samples. Figure 10 Which sample formed from lava that cooled rapidly? 1. A 3. C 2. E 4. D -------------- 42. Which granite sample most likely formed from magma that cooled and solidified at the slowest rate? 1. 2. 3. 4. -------------- 43. Which rock most probably formed directly from lava cooling quickly at Earth’s surface? 1. 3. 2. 4. -------------- 44. The graph below shows the relationship between the cooling time of magma and the size of the crystals produced. Which graph correctly shows the relative positions of the igneous rocks granite, rhyolite, and pumice? 1. 3. 2. 4. -------------- 45. Which sedimentary rock would be formed by the compaction and cementation of rounded pebbles? 1. shale 3. conglomerate 2. sandstone 4. siltstone -------------- 46. Which rock was most likely formed from pebble-sized sediment deposited in shallow water at an ocean shoreline? 1. shale 3. siltstone 2. basalt 4. conglomerate -------------- 47. Which sedimentary rock is composed of fragmented skeletons and shells of sea organisms compacted and cemented together? 1. shale 3. sandstone 2. limestone 4. gypsum -------------- 48. The diagram represents a conglomerate rock. Some of the rock particles are labeled. Which conclusion is best made about the rock particles? 1. 2. 3. 4. They are the same age. They originated from a larger mass of igneous rock. They all contain the same minerals. They have different origins. -------------- 49. [Refer to figure 1 in question 3] Which sample is composed of sediments 0.006 centimeter to 0.2 centimeter in size that were compacted and cemented together? 1. conglomerate 3. gneiss 2. sandstone 4. granite -------------- 50. Which rocks would most likely be separated by a transition zone of altered rock (metamorphic rock)? 1. 2. 3. 4. sandstone and limestone granite and limestone shale and sandstone conglomerate and siltstone -------------- 51. Which kind of bedrock would most likely contain fossils? 1. a high-grade metamorphic rock layer made from mixed igneous and sedimentary layers 2. a series of alternating layers of shale and sandstone 3. a basalt lava flow from an ancient volcano 4. a mass of granite in the core of a mountain -------------52. Which statement best describes pebbles? 1. 2. 3. 4. Pebbles are rocks that form due to melting and solidification. Pebbles are rocks that form due to cementation and compaction. Pebbles are sediments that weather from larger sand grains Pebbles are sediments that range in size from 0.2 cm to 6.4 cm. -------------- 53. Which characteristic is most common in sedimentary rocks? 1. 2. 3. 4. foliation layering intergrown crystals glassy texture -------------- 54. Which rock type most often contains fossils? 1. gabbro 3. limestone 2. quartzite 4. metaconglomerate -------------- 55. The sequence of diagrams below represents the gradual geologic changes in layer X , located just below Earth’s surface. Which type of sedimentary rock was formed at layer X? 1. conglomerate 3. rock salt 2. shale 4. coal -------------- 56. Base your answer on the cross section and data table. The cross section shows a sedimentladen river flowing into the ocean. The arrows show the direction of river flow. Different zones of sorted sediments, A, B, C, and D, have been labeled. Sediments have been taken from these zones and measured. The data table shows the range of sediment sizes in each zone. Figure 11 The sedimentary rock, siltstone, will most likely form from sediments deposited in zone 1. A 3. C 2. B 4. D -------------- 57. Which type of rock most likely contains fossils? 1. scoria 3. schist 2. gabbro 4. shale -------------- 58. Which pair must represent atoms of the same element? 1. and 2. and 3. and 4. and -------------- 59. Which substance is composed of atoms that all have the same atomic number? 1. magnesium 3. ethane 2. methane 4. ethene -------------- 60. All samples of an element are composed of atoms with the same 1. atomic mass 3. number of nucleons 2. atomic number 4. number of neutrons -------------- 61. 66. In a sample of the element potassium, each atom has Neutral atoms of 35Cl and 37Cl differ with respect to their number of 1. 2. 3. 4. 19 protons 20 neutrons 39 protons and neutrons 39 neutrons 1. electrons 3. neutrons 2. protons 4. positrons -------------- 67. -------------- What is the total number of electrons present in an 62. atom of What is the symbol for an atom containing 20 protons and 22 neutrons? ? 1. 27 3. 39 2. 32 4. 86 -------------- 1. 68. 2. 3. Which particles account for most of the mass of the atom? 4. -------------63. 1. protons and neutrons 3. neutrons and electrons 2. protons and 4. neutrons and electrons Which symbol represents an isotope of carbon? positrons -------------- 69. How many protons are in the nucleus of an atom of beryllium? 1. 2. 3. 1. 5 3. 9 2. 2 4. 4 4. -------------70. -------------- 64. Compared to the entire atom, the nucleus of the atom is Which statement best describes an electron? 1. It has a smaller mass than a proton and a negative charge. 2. It has a smaller mass than a proton and a positive charge. 3. It has a greater mass than a proton and a negative charge. 4. It has a greater mass than a proton and a positive charge. 1. smaller and contains most of the atom’s mass 2. smaller and contains little of the atom’s mass 3. larger and contains most of the atom’s mass 4. larger and contains little of the atom’s mass -------------71. All isotopes of a given element must have the same -------------65. What is the mass number of an atom which contains 28 protons, 28 electrons and 34 neutrons? 1. 28 3. 62 2. 56 4. 90 -------------- 1. atomic mass 3. mass number 2. atomic number 4. number of neutrons -------------- 72. The total number of electrons in a neutral atom of every element is always equal to the atom’s 1. mass number 3. number of protons 2. number of neutrons 4. number of nucleons -------------73. An atom that contains 8 protons, 8 electrons, and 9 neutrons has 1. an atomic number of 9 3. a mass number of 17 2. an atomic number of 16 4. a mass number of 25 -------------- Answer Key for Atoms Rocks Minerals 1. 4 26. 2 51. 2 2. 1 27. 1 52. 4 3. 3 28. 4 53. 2 4. 2 29. 1 54. 3 5. 3 30. 4 55. 4 6. 4 31. 4 56. 3 7. 3 32. 2 57. 4 8. 1 33. 3 58. 2 9. 2 34. 1 59. 1 10. 3 35. 3 60. 2 11. 4 36. 4 61. 1 12. 2 37. 1 62. 1 13. 1 38. 1 63. 3 14. 1 39. 3 64. 1 15. 1 40. 3 65. 3 16. 1 41. 3 66. 3 17. 2 42. 4 67. 1 18. 3 43. 2 68. 1 19. 2 44. 1 69. 4 20. 1 45. 3 70. 1 21. 3 46. 4 71. 2 22. 3 47. 2 72. 3 23. 3 48. 4 73. 3 24. 1 49. 2 25. 3 50. 2