neutralization practice
advertisement

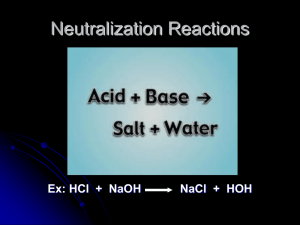
NEUTRALIZATION Chemical reactions occur when two or more substances are mixed together and they react with one another to form new substances. A chemical reaction is shown by writing a chemical equation, such as: HCl + NaOH → NaCl + H2O. The substances on the left of the arrow are called reactants and the substances on the right of the arrow are called products. Although it is a chemical equation, the arrow does not mean “equals”, it means “produces”. “produces” Reactants Products Neutralization is a chemical reaction, also called a water forming reaction, in which an acid and a base or alkali react and produce a salt and water. In other words, you can say that neutralization is the combination of hydrogen ions H+ and hydroxide ions OH− to form a water molecule, H2O. In the process, a salt is formed. Neutralization is exothermic, meaning it produces heat. Most generally, the following occurs: acid + base → salt + water For example, the reaction between hydrochloric acid and sodium hydroxide: Hydrochloric acid + Sodium hydroxide → Sodium chloride + water HCl + NaOH → NaCl + H2O Since the HCl and NaOH dissociate into ions in solution, the ionic equation is: H+ + Cl− + Na+ + OH− → Na+ + Cl− + H2O And since the sodium and chloride ions are just spectator ions not involved in the reaction, the net equation becomes: H+ + OH− → H2O This illustrates why neutralization reactions are also referred to as water forming reactions. Of course the sodium and chloride ions are still in solution so the result is pH neutral salt water. In addition, carbonates are compounds such as CaCO3, calcium carbonate (egg shell, chalk, oyster shells, limestone, etc.), and NaHCO3, sodium bicarbonate (baking soda). Acids will react with carbonate compounds to produce a salt, water and carbon dioxide. How vigorously they react will depend on the identity of the acid and of the carbonate, on the concentration of the acid, on the particle size of the carbonate, and other factors. As you can see, the acid is still removed, so the pH is changed in the products. Would the products be more acidic, or more neutral? Calcium carbonate + acetic acid (vinegar) Calcium acetate + water + carbon dioxide CaCO3(s) + 2 CH3COOH(aq) → Ca(CH3COO)2(aq) + H2O + CO2(g) QUESTIONS: What happens in a chemical reaction? ____________________________________________________ ___________________________________________________________________________________________. What are the substances on the left of the arrow called? _______________________________. What are the substances on the right of the arrow called? _______________________________. What does the arrow mean? ____________________________________________________________. What two substances are the reactants in a neutralization reaction? _________________________ and ____________________________. What two substances are the products in a neutralization reaction? _________________________ and _____________________________. What else is produced in a neutralization reaction besides water and salt? __________________________. When an acid reacts with CARBONATES, what is the product that you can see being made? _________________ Neutralization equations practice: 2 HCl + Mg(OH)2 MgCl2 + 2 H2O HCl + NaOH Al(OH)3 + HCl KOH + HBr