Synthesis of sulphamide linked dinucleotide analogues
advertisement

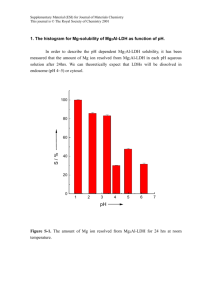
Supplementary Material (ESI) for Chemical Communications This journal is © The Royal Society of Chemistry 2000 ELECTRONIC SUPPLEMENTARY INFORMATION (ESI) Unusual RNA and DNA binding properties of a novel pyrrolidine–amide oligonucleotide mimic (POM) David T. Hickman,a Paul M. King,b Matthew A. Cooper,c Jonathan M. Slater,b and Jason Micklefield*a a Department of Chemistry, UMIST, Faraday building, PO Box 88, Manchester M60 1QD, UK. b Department of Chemistry, Birkbeck College, University of London, Gordon House, 29 Gordon Square, London WC1H 0PP, UK. c University Chemical Laboratory, Lensfield Road, Cambridge, CB2 1EW, UK. Dr M. A. Cooper UV thermal denaturation curves, Job plots and SPR sensograms for T 5– POM 3 binding to DNA and RNA. Supplementary Material (ESI) for Chemical Communications This journal is © The Royal Society of Chemistry 2000 2 1.15 Abs (260nm) 1.1 1.05 fast heating 1 slow cooling slow heating 0.95 0.9 0.85 0.8 15 25 35 45 55 65 75 85 95 temperature / deg C Figure 1. Variation of Absorption (A260) with temperature curves for an equimolar mixture of T5-POM 3 and Poly(rA) (42 M each in bases) in buffer A (10 mM K2HPO4 adjusted to 120 mM K+, pH 7.0) for a typical cycle of fast heating (5 ˚C/min), slow cooling (0.2 ˚C/min) and slow heating (0.2 ˚C/min). The large hysteresis between the slow heating and cooling curves indicates that the rate of association or dissociation is less than the rate of heating/cooling such that equilibrium between 3 and Poly(rA) is not attained. Typically at a heating/cooling rate of 0.2 ˚C/min little or no hysteresis is observed for native duplex forming oligonucleotides, suggesting that 3 and Poly(rA) associate and dissociate slowly compared with the hybridisation of native nucleic acids. This cycle of heating/cooling was used for determining Tms except where stated otherwise. Supplementary Material (ESI) for Chemical Communications This journal is © The Royal Society of Chemistry 2000 3 1 Abs (260nm) 0.95 5 degC/min 2 degC/min 1 degC/min 0.5 degC/min 0.1 degC/min 0.9 0.85 0.8 0.75 0.7 15 25 35 45 55 65 75 85 temperature / degC Figure 2. UV melting curves for T5-POM 3 and Poly(rA) (42 M each in bases) in buffer A following fast heating (5 ˚C/min) then cooling and heating at different rates (5, 2, 1, 0.5 and 0.1 ˚C/min). The larger hyperchromic shifts at slower rates of heating are presumably a consequence of the greater extent of duplex association and dissociation during the cooling and heating cycles respectively. Supplementary Material (ESI) for Chemical Communications This journal is © The Royal Society of Chemistry 2000 4 50 49 Tm / degC 48 47 46 45 44 43 0 1 2 3 4 5 Rate of heating (degC/min) Figure 3. Plot of Tm vs rate of heating/cooling (from figure 2). This shows that the observed Tm for T5-POM 3 and Poly(rA) is higher at lower rates of heating/cooling. By extrapolating to an infinitely slow rate of heating/cooling the true equilibrium Tm was determined to be 49˚C. Supplementary Material (ESI) for Chemical Communications This journal is © The Royal Society of Chemistry 2000 5 normalised Abs (260nm) 1.3 1.25 Poly (rU) Poly (rC) Poly (rG) Poly (rA) 1.2 1.15 1.1 1.05 1 0.95 15 25 35 45 55 65 75 85 95 temperature / deg C Figure 4. UV thermal denaturation curves for T5-POM 3 and an equimolar amount (42M each strand in bases) of Poly(rA), (rU), (rC) or (rG) in buffer A. The absence of significant hyperchromicity with non-complementary RNAs suggests that T5-POM binds sequence specifically to Poly(rA). n o r m a lis e d A b s ( 2 6 0 n m ) Supplementary Material (ESI) for Chemical Communications This journal is © The Royal Society of Chemistry 2000 6 1 .3 1 .2 5 1 .2 120m M 220m M 620m M 1200m M 1 .1 5 1 .1 K+ K+ K+ K+ 1 .0 5 1 0 .9 5 0 .9 15 25 35 45 55 65 75 85 95 te m p e r a tu r e / d e g C Figure 5. UV thermal denaturation curves of T5- POM 3 and Poly(rA) (42 M each in bases, 10 mM K2HPO4, pH 7.0) at different ionic strengths. An increase in ionic strength is accompanied by a moderate increase in Tm. The lower hyperchromic shifts observed at higher ionic strength are probably due to slower rates of association/dissociation of T5-POM with Poly(rA). Supplementary Material (ESI) for Chemical Communications This journal is © The Royal Society of Chemistry 2000 7 normalised Abs (260nm) 1.5 1.4 pH 6.0 pH 6.5 pH 7.0 pH 7.5 pH 8.0 1.3 1.2 1.1 1 0.9 25 35 45 55 65 75 temperature / degC Figure 6. UV thermal denaturation curves of T5- POM 3 and Poly(rA) at different pHs (42 M each in bases, 10 mM K2HPO4, 120 mM K+). Considerably higher Tm values and larger hyperchromic shifts are observed at lower pH. The later is a reflection of faster association at lower pH such that during the annealing cycle a higher proportion of strands have hybridised. Supplementary Material (ESI) for Chemical Communications This journal is © The Royal Society of Chemistry 2000 8 60 58 Tm / degC 56 54 52 50 48 46 44 42 40 6 6.5 7 7.5 8 pH Figure 7. Plot of Tm vs pH for T5-POM 3 and Poly(rA) derived from figure 6. The most marked change in Tm occurs between pH 6.5 - 7.0. Supplementary Material (ESI) for Chemical Communications This journal is © The Royal Society of Chemistry 2000 9 0.6 0% A 10% A 20% A 30% A 40% A 50% A 60% A 70% A 80% A 90% A 100% A 0.5 Abs 0.4 0.3 0.2 0.1 0 220 230 240 250 260 270 280 290 300 Wavelength / nm Figure 8. UV spectra of T5-POM 3 and Poly(rA) at different molar ratios. Mixtures of 3 and Poly(rA) 50 M total base concentration in buffer A were allowed to anneal for 24 hr at 25 ˚C before UV spectra were recorded. The lowest absorbance maximum in the range 255–275 nm is obtained at a 1:1 (T:A) molar ratio indicative of duplex formation. 0.6 Supplementary Material (ESI) for Chemical Communications This journal is © The Royal Society of Chemistry 2000 10 Figure 9. A Job plot of T5-POM 3 and Poly(rA) showing UV maximum absorbance (Amax) in the 255–275 nm region vs molar ratio derived from figure 8. Job plots of A260 vs molar ratio also show a minima at a 1:1 (T:A) molar ratio, but the minima is less well defined due to the large difference in max for poly(rA) (257 nm) and 3 (272 nm). Supplementary Material (ESI) for Chemical Communications This journal is © The Royal Society of Chemistry 2000 11 1.2 normalised Abs (260nm) 1.18 1.16 1.14 1.12 1.1 1.08 1.06 1.04 1.02 1 15 25 35 45 55 65 75 85 95 temperature / degC Figure 10. Melting curve for T5-POM 3 and Poly(dA), 120 mM K+, pH 7 at an heating rate of 0.2 ˚C/min. The samples were incubated for 48 h at a fivefold increased concentration (210 M each in bases) prior to dilution to 42 M as described in the main text. Even after such a long incubation period it is likely that only a fraction of the complex has formed. Supplementary Material (ESI) for Chemical Communications This journal is © The Royal Society of Chemistry 2000 Figure 11. Melting curve for T5-POM 3 and Poly(dA), 1200 mM K+, pH 7.0. 12 Supplementary Material (ESI) for Chemical Communications This journal is © The Royal Society of Chemistry 2000 13 normalised Abs (260nm) 1.026 1.022 1.018 1.014 1.01 1.006 1.002 0.998 15 25 35 45 55 65 75 85 95 temperature / degC Figure 12. Melting curve for T5-POM (3) and Poly(dA) 620 mM K+, pH 7.0, after 96 h incubation . Although the absorbance change is small, two hyperchromic shifts are observed consistent with melting of a triplex to a duplex and then melting of a duplex to single strands. Supplementary Material (ESI) for Chemical Communications This journal is © The Royal Society of Chemistry 2000 14 n o rm alised Ab s (260n m ) 1.1 1.08 1.06 1.04 1.02 1 0.98 15 25 35 45 55 65 75 85 95 tem perature / degC Figure 13. Melting curve for T5-POM (3) and Poly(dA) at 0.2 ˚C/min, 120 mM K+, pH 6.0 after 96 h incubation. Again two hyperchromic shifts are observed indicative of triplex denaturation. Supplementary Material (ESI) for Chemical Communications This journal is © The Royal Society of Chemistry 2000 15 0.7 0.6 0% A 10% A Abs 0.5 30% A 40% A 0.4 50% A 60% A 0.3 80% A 0.2 90% A 100% A 0.1 0 220 230 240 250 260 270 280 290 300 wavelength / nm Figure 14. UV spectra T5-POM 3 and Poly(dA) at different molar ratios. Mixtures of 3 and Poly(rA) 210 M total base concentration in buffer A were allowed to anneal for 48 hr at 25 ˚C, then diluted with buffer A to a total base concentration of 42 M, before UV spectra were recorded. The lowest absorbance maximum in the range 257–272 nm is obtained at 30% Poly(dA), a 2:1 (T:A) molar ratio, indicative of triplex formation. (Scans of 20 % and 70 % Poly(dA) have been omitted to improve clarity). Supplementary Material (ESI) for Chemical Communications This journal is © The Royal Society of Chemistry 2000 16 0.58 0.56 0.54 Abs max 0.52 0.5 0.48 0.46 0.44 0.42 0.4 0 10 20 30 40 50 60 70 80 90 100 mol % Polyd(A) Figure 15. A Job plot of T5-POM 3 and Poly(dA) showing UV maximum absorbance (Amax) in the 257-272 nm region vs molar ratio derived from figure 14. Supplementary Material (ESI) for Chemical Communications This journal is © The Royal Society of Chemistry 2000 17 Figure 16. SPR response (RU) vs. time for T5-POM 3 injected across r(A)20 (), d(A)20 (), d(AGC TTC AGA GAT CGA TCG GAG AGA GTA GTG–3´) () derivatised surfaces and an underivatised control surface (). Response profiles were determined using a BIAcore 2000 instrument (Biacore AB, UK) under standard conditions following injection of T5-POM 3 (40M strand concentration, 10 mM K2HPO4, pH 7 adjusted to 0.12 M K+) across each surface at a flow rate of 20 L/min for 300 sec followed by buffer to allow dissociation. All oligonucleotides were biotinylated at the 5´–end (Cruachem, UK) and immobilised on a streptavidin derivatised carboxymethyl dextran sensor chip (SA) (Biacore). Supplementary Material (ESI) for Chemical Communications This journal is © The Royal Society of Chemistry 2000 18 700 600 500 400 RU 300 200 100 0 -100 -100 100 300 500 700 t / sec Figure 17. SPR response (RU) vs. time for T5-POM 3 injected across r(A)20 (), d(A)20 (), d(AGC TTC AGA GAT CGA TCG GAG AGA GTA GTG–3´) () derivatised surfaces and an underivatised control surface (). Response profiles were determined as described in figure 16 except 80M strand concentration of T5-POM 3 was injected across each surface.