lesson
advertisement

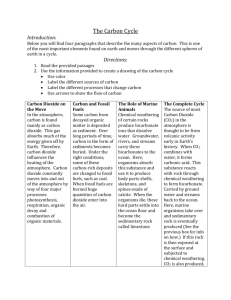
Carbon Dioxide—Where Can We Find It? TITLE: Carbon Dioxide—Where Can We Find It? SUBJECT/TOPIC: Carbon dioxide (CO2) and the carbon cycle GRADES: 9–12 STANDARDS ALIGNMENT: Science as Inquiry Earth and Space Science-Geochemical cycles Science in Personal and Social Perspectives TIME ALLOWANCE: Two 50-minute periods OVERVIEW AND PURPOSE / OBJECTIVE(S) (information, concepts to be learned): Students will set up experiments to help them better understand CO2 and its presence and impact on the carbon cycle and the environment. 1. Students will be able to explain and test for sources of CO2. 2. Students will understand the use of an indicator solution (limewater) to reveal the presence of CO2. 3. Students will understand the importance of carbon dioxide. Vocabulary: Deforestation—the removal of a forest or stand of trees where the land is thereafter converted to a non-forest use. Fossil fuel—there are three major forms of fossil fuels: coal, oil, and natural gas. All three were formed many hundreds of millions of years ago before the time of the dinosaurs; hence the name fossil fuels. Fossil fuels are derived from the pressurization of plants over long periods of time. Greenhouse gas—gases that trap heat in the atmosphere are often called greenhouse gases. Greenhouse gases such as carbon dioxide occur naturally and are emitted to the Carbon Dioxide—Where Can We Find It? (High School) 1 atmosphere through natural processes and human activities. Other greenhouse gases (e.g., fluorinated gases) are created and emitted solely through human activities. Source—anything that releases CO2 into the atmosphere. Sink—anything that absorbs and holds CO2 from the atmosphere or water. Materials: For each two-student team test tube rack three test tubes with stoppers (one for each source of carbon dioxide) one test tube with a one hole stopper for Part 1—using limewater coffee filters or filter paper (for making limewater solution) clean container for storing limewater solution markers safety goggles straws student worksheet Note: other materials may be needed, depending upon the student sources of carbon dioxide. Activity/Engagement: (reinforcing lesson, making real-world connection) Carbon dioxide (CO2) is a greenhouse gas. Carbon dioxide is important because it keeps the Earth’s average temperature at about 15 °C (59 °F), making it habitable for humans and many living things. The CO2 traps infrared energy emitted from the Earth’s surface and warms the atmosphere. Without water vapor, CO2, and methane (the three most important naturally produced greenhouse gases), the Earth’s surface temperature would be about −18 °C (0 °F). At this temperature, most life cannot survive. Where does CO2 come from? Plants and animals give it off when they extract energy from their food during cellular respiration. Carbon dioxide bubbles out of the earth in soda springs, explodes out of volcanoes, and is released when organic matter burns (such as during forest fires). Carbon Dioxide—Where Can We Find It? (High School) 2 Figure: The Carbon Cycle From library.thinkquest.com In today’s atmosphere, CO2 levels are climbing in a dramatic and easily measurable fashion, providing evidence that there is now more CO2 than ever before. What are the sources for this additional CO2? Human activities are thought to be primarily responsible for the observed increases. The major human sources of CO2 are as follows: Fossil fuel combustion (the combustion in a car engine, for example) accounts for 65%. Deforestation (CO2 released from trees that are cut and burned or left to decay) accounts for 33%. Deforestation also decreases the amount of oxygen given off by plants. The by-products of cement production account for the remaining 2%. The natural biological and geological sources mentioned above are insignificant contributors to CO2 when compared to the human sources. Plants (both terrestrial plants and marine phytoplankton), which use carbon dioxide for photosynthesis, are the most important ways in which carbon dioxide is taken out of the atmosphere. Atmospheric CO2 can also be dissolved directly into ocean waters and thereby be removed from the atmosphere. Although plants also release CO2 through the process of respiration, on a global, annual basis, the amount of CO2 used by plants through photosynthesis and released through respiration approximately balances out. Scientists typically monitor the concentration of CO2 in atmospheric samples by using sensitive devices called infrared gas analyzers. These devices pass a beam of infrared (IR) light through a sample of gas. The amount of IR that reaches a detector on the other side can be used to determine the amount of CO2 in the sample. A worldwide network of CO2 monitoring stations currently tracks the Earth’s rising CO2 levels. Carbon dioxide has another characteristic that enables students to detect CO2 themselves. Carbon Dioxide—Where Can We Find It? (High School) 3 In this activity, students will be testing possible sources of carbon dioxide. In Part 1, students will understand the process of detecting carbon dioxide by using limewater. When carbon dioxide is bubbled into limewater, calcium carbonate (CaCO3) is produced. It precipitates out as a white suspended solid, making the solution appear cloudy. Preparation of limewater: Put 1 teaspoon of calcium hydroxide in a clean glass jar, up to 1 gallon in size. (Limewater is a saturated solution, which means there will be some extra chemical that doesn’t dissolve. A teaspoon will result in a fully saturated solution whether you use a gallon jar or a smaller one.) Fill the jar with distilled or tap water. Shake the jar vigorously for 1–2 minutes, then let it stand for 24 hours. Pour the clearer solution off the top of the jar through a clean coffee filter or filter paper, being careful not to stir up the sediment. Repeat the filtering step if necessary to obtain a clear limewater solution. Store in a clean jar or bottle. (From http://www.hometrainingtools.com/makinglimewater-solution-science-teaching-tip/a/1101/.) In Part 2, students will collect and detect CO2 from three sources and test for carbon dioxide. Note: For the activity to be most effective, students should have a working knowledge of the carbon cycle (see Figure). Part 1: Detecting Carbon Dioxide Gas In the first part of the experiment, students in teams of two should experience what carbon dioxide does to limewater as a sample test. Use the following procedure: 1. Have students put 10–20 ml of limewater into a test tube with a one-hole stopper. 2. Place a straw through the one-hole stopper and breathe into the limewater. 3. What happens to the limewater? After the students have completed this test, discuss with them some other ways that limewater can be used as a test (leave sample opened, bubble something through it, etc.). Discuss these methods with your class. Carbon Dioxide—Where Can We Find It? (High School) 4 Part 2: Three Sources of CO2 Ask your students to design an experiment, using limewater, to detect the carbon dioxide from three sources. Some sources might be the reaction of vinegar and baking soda; a sprig of elodea or other plant material; dry ice; engine exhaust (see note below). Ask students to work in teams of two and discuss where they might be able to locate carbon dioxide. Ask them to write three sources on their student worksheet. The students will then test their ideas by using limewater (see preparation above) to see if carbon dioxide is present. They can write their hypothesis on the worksheet. Students must design their experiment by using the worksheet in this activity. A teacher must sign off on their design before they begin their experimentation. Note: Students SHOULD NOT collect automobile exhaust. If the class decides that this is a source to be examined, and the teacher approves, instructions for the teacher to collect exhaust can be found at http://www.ucar.edu/learn/1_4_2_17t.htm. Concluding Discussion/Activities: This activity lends itself to follow-up opportunities—ask students questions such as the following: What are some other sources of CO2 that you did not test? If you wished to reduce the amount of CO2 increase in the atmosphere, which source would be most important to control? Explain why. Would there be problems with such controls? If so, what might they be? Discuss the amounts of CO2 produced by each source. How could this be used to describe amounts of CO2 present today? In looking at the sources of CO2 in this investigation, how could the amount of CO2 be decreased? Carbon Dioxide—Where Can We Find It? (High School) 5 Student Worksheet for Carbon Dioxide—Where Can We Find It? Experiment Title: __________________Date: _____ Name: ______________________ Student Hypothesis: ______________________________________________________ Sources of CO2 : Materials Needed for This Lab: Safety Precautions: ________________________________________________________________ ________________________________________________________________ Procedure: Lab safety equipment should be used, and safety protocols followed. _______________________________________________________________ _______________________________________________________________ _______________________________________________________________ _______________________________________________________________ _______________________________________________________________ _______________________________________________________________ _______________________________________________________________ ______________________________________________________________ _______________________________________________________________ _______________________________________________________________ Teacher signature on lab design___________________________________ Carbon Dioxide—Where Can We Find It? (High School) 6 Data and Observations: Source #1 Source #2 Source #3 Carbon Dioxide—Where Can We Find It? (High School) 7