Experiment 1 Pipetting
advertisement

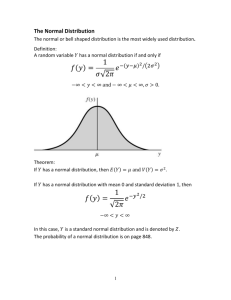
BIOCHEMISTRY 3723 EXPERIMENT 1 PIPETTING Report Author: Lab Partner: Date Due: Date Turned In: Procedural Changes: Indicate any changes you made from the procedure in the lab manual. Explain why these changes were made and how they affect the results. Results: 1. Use of Glass Pipets: a. Fill in the Table 1 below with the data obtained using the glass pipets. Table 1: Glass Pipets Pipet used Weight of beaker before add water Weight of Weight of beaker with water water Volume of water 5.00 mL pipet: 1. 1. 1. 1. 2. 2. 2. 2. 3. 3. 3. 3. 1. 1. 1. 1. 2. 2. 2. 2. 3. 3. 3. 3. 3.00 mL water 10.00 mL pipet: 7.50 mL water Average volume of water b. Calculate your accuracy (mean % absolute error) with each pipet, using the intended volume (i.e. 3.00 mL for the 5.00 mL pipet) as the theoretical value and your average volume as the experimental value in the formula: mean % absolute error = Average Expt' l Value - Theoretical Value 100% Theoretical Value Record accuracy in Table 2. Show a sample calculation below. Note: The absolute error may be positive or negative. c. Calculate the precision of your pipetting (mean % deviation) using the formula: n x i 1 i x 100% nx where xi = the individual experimental value x = the average of all experimental values n = the number of experimental values Record values in Table 2. Show a sample calculation below. mean % deviation = Table 2: Accuracy and Precision of Glass Pipets Pipet used: Theoretical Volume 5 mL: 3.00 mL Average Volume Measured Accuracy (mean % absolute error) Precision (± mean % deviation) % ± % % ± % 10 mL: 7.50 mL The difference between precision and accuracy is that accuracy relates a measured value to the true known value and precision relates an individual measured value to the average of all like measurements. 2. Use of Digital Micropipetters: a. Fill out Table 3 below with the data obtained for the micropipetter. Table 3: Micropipetter Pipetter Number and Size Volume in pipet: < 90, = 90, or > 90 µL 1. 2. 3. Total volume of water Average total volume of water 1. 2. 3. b. Calculate accuracy and precision of measurements with the micropipet as done above for glass pipets. (Use 90.0 µL as the theoretical volume, and show work). 3. A375 of replicate K2CrO4 dilutions: Calculation of standard deviation a. Record the A375 values in Table 4. Calculate and record the average (mean) absorbance value, x . Table 4: K2CrO4 Calibration of Spectrophotometer # A375 Av. A375 Precision (Std. Dev., s) % relative error mean % absolute error 1 2 3 4 5 6 7 8 b. A better measure of the precision of an experimental determination than the one used above is the standard deviation of the sample (s). To get a good estimate of standard deviation, you need at least six replicates of the determination. Standard deviation of a sample is defined by the formula: n x i sx x 2 i 1 n 1 It is easier to work with one of two computing forms of this formula: 2 n n n 2 n xi x i x 2i nx 2 i 1 i1 sx i 1 nn 1 n 1 Calculate the standard deviation (s) and enter it in Table 4. If using a calculator, make sure you are calculating s and not . Also calculate % relative error of the standard deviation and enter it in Table 4. s s = % relative error = x 100% x [Note: Standard deviation of the value (s) differs slightly from standard deviation of the population () See any statistics text]. c. Alkaline solutions of K2CrO4 are used to calibrate the absorbance scales of spectrophotometers, because these solutions are very stable and their absorbance is very reproducible. The dilutions you made are predicted to have A375 = 0.400. Determine the accuracy of your determination by computing % absolute error of your mean experimental value from this theoretical value. Enter value in Table 4. Show calculations. d. Discuss briefly what you think are the main causes of the deviation that limited the precision of your replicate determinations of A375 of a 1/30.0 dilution of the standard K2CrO4 solution. If you think you could decrease this error, explain how. e. Discuss briefly what you think are the main causes of the absolute error that limited the accuracy of your average determination of A375 of a 1/30.0 dilution of the standard K2CrO4 solution. If you think you could decrease this error, explain how. f. Indicate the number of significant figures with which each of the following measurement in part C was made: weight of K2CrO4, measurement of K2CrO4 solution, measurement of diluent, and A375 readings. What is the appropriate number of significant figures for the determination of the precision (s) and for the determination of the accuracy (% absolute error)?