Linking Met Receptor Signaling to Cell Polarity INTRODUCTION
advertisement

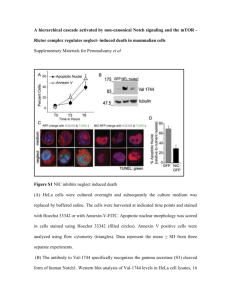
Linking Met Receptor Signaling to Cell Polarity INTRODUCTION Deregulation of the Met receptor, a receptor tyrosine kinase, causes cells to exhibit phenotypes reminiscent of metastatic or tumor cells [1]. Such phenotypes include cell invasion, cell proliferation, cell survival and cell migration. In order for a tumor to become metastatic its cells need to acquire the ability to migrate so as to invade other areas of the organism. However, the molecular details leading to the cellular changes required for cell migration downstream of receptor tyrosine kinases still need to be elucidated. Recently, Dr. Park’s laboratory has identified, by co-immunoprecipitation and subsequent mass spectrometry, interactors of the Gab1 scaffold protein, which earlier was determined to be essential for propagating the Met signaling response [2]. Given that these interactor proteins communicate with Gab1 it is plausible that they play a role in Met signaling. Included in the interactors of Gab1 was the CLIP associating protein, Clasp1. Humans have two Clasp homologs, Clasp1 and Clasp2, both of which are plus-end microtubule binding proteins. Clasp proteins have been shown to play roles in regulating cellular migration by stabilizing the subsets of microtubules oriented towards the leading edge of migrating cells [3]. Clasp1 and Clasp2, from now on referred to as Clasp1/2, share 69.7% sequence identity conferred by Multiple Sequence Alignment and have been shown to play partially redundant functions in microtubule stabilization [4, 5] and proper chromosomal segregation [6, 7]. Given the common functionality of both Met receptor signaling and Clasp proteins in cell migration, Clasp proteins seem like promising candidates in transducing the signals from the Met receptor into a modulation of the microtubule cytoskeleton allowing cell migration to occur. Loss of heterozygosity in a region of chromosome 3p, where the Clasp2 locus is situated, is a hallmark of mammary carcinoma and non-small cell lung cancer [8, 9]. Moreover, low levels of Clasp2 mRNA expression correlate with poor prognosis and higher tumor grade [2, 10]. Similarly, low levels of Clasp2 mRNA transcripts are seen in breast tumors compared to normal tissue [10]. In contrast, higher levels of Clasp2 are evident in cases with a better patient prognosis [10]. The depressed levels of Clasp2 associated with a more malevolent cancer intimate a potential tumor suppressor role for Clasp2 in pulmonary and mammary carcinoma. Often upregulation of an oncogene (e.g. Met receptor) is accompanied by downregulation of a tumor suppressor gene compounding the cancer’s ability of expropriating unaffected cells. Met signaling is also linked to breast cancer and non-small cell lung cancer [11, 12], suggesting that Clasp2 and Met may play a role in the same milieu and that deregulation of Clasp2 could contribute to Met’s role in oncogenesis. To investigate Clasp’s role in the Met signaling response it was necessary to 1) confirm the interaction with Gab1 observed in the laboratories initial experiments and 2) observe the effect of activating Met on Clasp’s localization in the cell. In order to accomplish this, immunoprecipitation, Western blot analysis, immunoflourescence and live-cell imaging were employed. Optimization of the expression and detection of GFP-Clasp2 proteins in HeLa cells was achieved, which will in subsequent studies, allow one to address if the Brady Eason Page 1 Gab1-Clasp2 interaction is dependent on Met signaling. Furthermore, I observed that GFPClasp2 changes its localization in response to Met activation was, suggesting that Clasp proteins are regulated by Met signaling. MATERIALS AND METHODS Cell Line Tissue Culture: HEK (human embryonic kidney) 293, MDCK (Madin-Darby Canine Kidney), HeLa and HeLa 299s (derived from human cervical cancer) cell lines were maintained in DMEM supplemented with 10% fetal bovine serum and 0.05 mg/mL Gentamycin. Cell lines were kept at 37°C in a humidified incubator with 5% CO2. Immunoprecipitation: Immunoprecipitations were performed using 500 µg of total lysate protein derived from cells that had been stimulated with 100 U/mL, 37°C HGF at the following time points: 120, 60, 30, 15, 5, 1, 0 (x2) minutes. One 0 minute time point was used as a negative control indicating protein levels prior to stimulation. The other 0 minute sample was incubated with no primary antibody to observe non-specific interactions, such as proteins binding to the resin themselves. Lysis of the cells was done using 1% Triton-X100 supplemented with 1mM PMSF, 10 µL/mL Aprotinin and Leupeptin and 1% Na3O4V acting as protease/phosphatase inhibitors. Two similar immunoprecipitations were performed in parallel – one immunoprecipitating GFP (i.e. using anti-α-GFP antibody as the primary) and the other immunoprecipitating HA (i.e. using Hemagluttin antibody as the primary). In both cases the quantity of primary antibody used was 1 µL stock in 250 µL of lysis buffer and the incubation occurred for 2 hours at 4°C on a nutator. The antibody was subsequently bound to 20 µL of a resin coated with Protein A or G, recognizing the constant part of rabbit or mouse IgG respectively. This incubation lasted one hour at 4°C on a nutator. Following three washing steps with 500 µL of lysis buffer and resuspension of the beads, the protein was loaded in a gel for Western blot analysis. Two input gels were also loaded with 30 µL of total lysate protein in each lane. The transfer of the gels occurred at 4°C onto a methanol activated membrane at 100 V for 1.5 hours. The membranes were blotted for many different proteins, including Erk/pErk and Met/pMet indicators of a successful HGF stimulation. Immunoflourescence: Immunoflourescence was performed in 24 well plates that contained sterilized cover slips coated with Poly-D-Lysin for improved cell adhesion. Stimulation at 15, 5, 2, 1 and 0 minutes with 100 U/mL, 37°C HGF and a subsequent 20 minute fixation with 4% paraformaldehyde were performed on stable GFP-Clasp2 expressing cell lines. Likewise the same protocol was used on a stable expressing GFP-Gab1 cell line for the purpose of a positive control. PBS was used for all wash steps, and all incubations steps were performed on a nutator. An anti-α-tubulin antibody was used to mark the microtubules and incubated for 1 hour prior to a respective secondary antibody incubation also lasting 1 hour. A DAPI stain to visualize the nucleus was incubated for 2 minutes and the cover slips washed with double distilled water two times before being mounted on microscopy slides using immuno-mount (Thermo Scientific, Pittsburgh). Lastly, the slides were allowed to dry overnight protected from light until viewing. Brady Eason Page 2 Live Cell Imaging: MDCK stable GFP-Clasp2 expressing cells were grown in 35 mm glassbottom dishes at 50,000 cells/plate. Prior to imaging, the cell medium was replaced with a prewarmed 1.5 mL Leibovitz CO2 independent medium to maintain proper pH throughout the experiment. Time-lapse movies of the cells kept at 37°C were taken using epiflourescence microscopy and a 63X objective. Pictures were taken every 1 minute for 35 cycles and then compiled in a time-sequence elapse manner. HGF of a final concentration of 100 U/mL was added between time points 4 and 7 to activate the MET response. RESULTS Confirming the Gab1-Clasp Interaction Upon Hepatocyte Growth Factor (HGF) stimulation of the Met receptor, its adaptor, Gab1, is recruited under the receptor, phosphorylated and involved in attracting many other proteins required for the signal transduction pathway. Since the lab has provided evidence that Gab1 and Clasp1/2 interact, as well as demonstrating that stimulation of the Met receptor with its ligand HGF causes cells to recapitulate the cancerous phenotypes mentioned above (importantly cell migration) it was only natural to suspect that the recruitment of Clasp1/2 is potentially dependent on stimulation. In the previous co-immunoprecipitation experiments the interaction between Gab1 and Clasp proteins was observed after transient expression of fusion proteins in a cell line derived from human embryonic kidney cells (HEK 293s). In these experiments, the interaction between GFP-Clasp1 and HA-Gab1 seemed to be independent of Met signaling. However, the HEK 293 cells contained only modest levels of endogenous Met receptor; suggesting that if the Gab1-Clasp1/2 interaction is enhanced upon Met activation this may have been undetectable in the previous experiments. We hence attempted to perform coimmunoprecipitation experiments in a cell line that exhibits higher levels of endogenous Met. HeLa cells are cervical cancer cells known for meeting this criterion. After co-transfecting HA tagged Gab1 and GFP tagged Clasp2 into HeLa cells, only low levels of GFP-Clasp2 expression was detected by Western blot. To address the problem, the quality and quantity of the DNA as well as the cell type and the antibody used for detecting GFP were optimized: poor transfection efficiency can be caused by inferior DNA quality, e.g. high content of nicked DNA versus supercoiled DNA. A Qiagen Midiprep kit was first used to freshly purify the GFP-Clasp1/2 DNA in an effort to improve its quality. The freshly prepared DNA was tested and compared to the previous GFP-Clasp1/2 DNA by using both HEK 293 and HeLa cells. Again, by Western blot analysis no GFP-Clasp1/2 was observed to be expressed at detectable amounts. However, a cell line stably expressing GFP was used as a positive control, and GFP was detected clearly which implies the transfection, lysis, loading, and transfer all were done properly. Next a Maxiprep using a CsCl gradient, which is a protocol used to produce very high quality plasmid (i.e. high ratio of supercoiled to nicked), was used to purify GFP-Clasp1/2 DNA. Concomitantly, another step taken to achieve better levels of GFP-Clasp1/2 expression was to use a different cell line, HeLa 299s, which is modified in such a way that it is more readily Brady Eason Page 3 transfectable than standard HeLa cells. As shown in Figure 1, transfection of the DNA from the Maxiprep CsCl preparation followed by Western blot of the whole cell lysate showed that indeed, GFP-Clasp1/2 are expressed at higher levels in HeLa 299s when compared to HeLa cells. Figure 1: Western blot showing DNA transfected in each lane (“-“ means not present whereas “+” means DNA was added to cells) as well as the protein expression detected with specific antibodies (labels on the right). One can see that GFP-CLASP1/2 is expressed at higher levels in the HeLa 299 than the standard HeLa cells. This is also evident for HA-Gab1 and endogenous Met. Low expression of exogenous proteins may also be the consequence of low expression from the promoter or impaired protein stability. In these cases, transfecting higher amounts of DNA can promote expression. In order to optimize expression levels various quantities of DNA were transfected. In addition, we also co-transfected pMX vector, which has been observed in the laboratory to “boost” expression of the co-transfected plasmid, possibly by acting as carrier DNA. Increasing quantities of DNA up to 625 ng/1.2x105 cells led to higher expression levels of GFP-Clasp2 protein (Figure 2). Likewise, co-transfection of pMX significantly increased expression levels. In future experiments, increased amounts and co-transfected pMX vector were used. Interestingly, lower levels of expression for GFP-Clasp2 compared to GFP-Clasp1 were observed. This is consistent with data from Akhamanova and colleagues [5]. It was shown by both Western blot analysis and immunoflourescence that the expression of GFP-Clasp2 is significantly less than that of GFP-Clasp1 in Cos-1 cells (Figure 5 and data not shown). This could be a contributing factor to the expression problems faced in Dr. Park’s lab with GFPClasp2 in the HeLa 299s. Brady Eason Page 4 Figure 2: Western blot showing different quantities of GFP-Clasp2 DNA transfected in HeLa 299s along with 1 µL of HAGab1. Lane 6 is equivalent to lane 3 except that lane 6 also had 1 µL of pMX vector cotransfected. It seems as though GFPClasp2 and HA-Gab1 potentiate each other’s expression levels. Seemingly low GFP-Clasp1/2 expression levels could also be attributed to problems with protein detection. To optimize GFP detection, various anti-GFP antibodies were tested. Six different anti-GFP antibodies were compared using whole cell lysates of B1-3 cells (a clone of MDCK cells stably expressing GFP-Gab1) with MDCK WT cells being used as a negative control in a Western blot analysis (Figure 3, left panel). The two anti-GFP antibodies that performed best in the Western blot analysis, AbCam and Molecular Probes 622086, were tested in an IP using stable MDCK expressing GFP-Clasp2 cells in order to ensure their suitability for biochemical approaches (Figure 4, right panel). Figure 3 & 4: (Left) Western blot analysis showing detection of GFP-Gab1 (black arrow) with 6 different GFP antibodies. The membrane was Ponceau-stained, cut longitudinally, washed, incubated in the individual antibodies and then reconstructed before detection so the exposure times would be equal. (Right) An immunoprecipitation experiment immunoprecipitating GFP-Clasp2 protein stable expressed in MDCK cells. Western blotting for GFP shows AbCam anti-GFP antibody detects as well as the molecular probes antibody but is more specific. FS stands for FACs sorted, which was done to select mid level GFP-Clasp2 expressing cells. Brady Eason Page 5 The Western blot analysis revealed that AbCam and Molecular Probes 622086 most efficiently detected the GFP-Gab1 protein, but the later detected more non-specific protein. This was significant for the lab because in the future AbCam anti-GFP antibody may be preferable to the Molecular Probes anti-GFP antibody currently being used. From the immunoprecipitation, similar results were observed - both detected the protein of interest at equivalent levels but the AbCam was more specific. Figure 5: Western blot analysis published in Akhmanova’s 2001 paper “CLASPs Are CLIP-115 and -170 Associating Proteins Involved in the Regional Regulation of Microtubule Dynamics in Motile Fibroblasts.” Left side shows detection of GFP-Clasp1/2 with anti-α-GFP antibody, whereas the right blot shows detection of GFP-Clasp1/2 with a Clasp2 specific antibody. After the optimization of expression and detection, multiple immunoprecipitation experiments with transiently expressing HA-Gab1 and GFP-Clasp2 were performed in HeLa 299s. Both immunoprecipitating GFP-Clasp2 and conversely HA-Gab1 were performed in these optimal conditions. Then the membranes were subsequently blotted for HA-Gab1 or GFP-Clasp2 respectively to observe the efficacy of the co-immunoprecipitation. The total cell lysates or inputs of each time point were blotted for GFP and HA to verify the expression of GFP-Clasp2 and HA-Gab1. Similarly, the inputs were blotted for Erk and pErk (a downstream protein involved in the Met signaling response typically used to measure HGF activation) to determine the success of the HGF stimulations. Total Erk levels were similar in all time points and pErk protein levels increased fairly uniformly with increased duration of HGF exposure as expected (Figure 6). Unlike the co-immunoprecipitation experiments validating the Gab1-Clasp1/2 interaction observed by the laboratories initial experiments in HEK 293 cells, the coimmunoprecipitations with the HeLa 299s did not corroborate such results. Successful coimmunoprecipitation of HA-Gab1 with Myc-Pak4, an established interactor of Gab1, was performed in parallel to the aforementioned immunoprecipitations and confirmed that the protocol was carried out correctly (data not shown). We therefore conclude that exogenously expressed Gab1 does not interact with Clasp2 in HeLa cells. Possible rationales for these outcomes are detailed in the discussion. Brady Eason Page 6 Figure 6: Immunoprecipitation of both GFP and HA done in parallel. Blots for HA-Gab1 and GFP-Clasp2 respectively are assessing the potential Gab1-Clasp interaction. Quantities of plasmid DNA are noted in the labels above the developed membranes. Proteins blotted for are found on the right side and the membranes blotted are listed on the left. Does Met Stimulation Cause Relocalization of GFP-Clasp2? Upon stimulation of the Met receptor, Gab1 is known to relocalize from the cytoplasm to the cortex of cells. Similarly, it has been shown that in wound-healing assays, GFP-Clasp2 localizes to the leading edge of cells [3, 5]. HA-Gab1 and GFP-Clasp1/2 interact in HEK 293 cells suggesting that Clasp1/2 might relocalize upon stimulation of the Met receptor as well. To address this question, live-cell imaging was performed using MDCK cells stably expressing GFP-Clasp2. Prior to Met stimulation GFP-Clasp2 exhibited a diffuse, continuous appearance in the cytosol (Figure 7). Upon HGF stimulation, GFP-Clasp2 was seen to take on a more filamentous appearance and single bright dots moving in the cytoplasm were detectable in timelapse (Figure 7). The filamentous localization may reflect binding to the microtubule lattice and the moving dots may reflect GFP-Clasp2 tracking microtubule plus ends (i.e. following the positive tip of a microtubule through its dynamic life of polymerizing rescues and depolymerizing catastrophes). Brady Eason Page 7 Figure 7: Time-lapse snapshots extracted from a video showing the affect of HGF stimulation on GFP-Clasp2 and its potential tracking of microtubules. Image 1 (top left corner) is without HGF. All other images have HGF present. Blown up images of the top two corner pictures are seen in the bottom row for comparison. It is also evident that there are concentrations of GFP-Clasp2 in some areas of the cortex (marked in yellow in image 4 but can be seen in the other images as well). In order to faithfully determine that GFP-Clasp2 localized to microtubules in response to HGF, MDCK GFP-Clasp2 stably expressing cells were fixed and stained with an anti-α-tubulin antibody at various time points post stimulation. In this experiment B1-3, stable MDCK GFPGab1 expressing cells where used as a positive control to verify whether or not the stimulation worked. In Figure 8 it is evident that the stimulation was successful because Gab1 relocalized to the cell cortices as early as one minute post-stimulation. Moreover, changes in GFP-Clasp2’s localization are apparent (Figure 9). With no stimulation the GFP signal is homogenous and smooth. Post-stimulation the GFP signal is seen to be more filamentous and non-uniform. In some cases, a high concentration is seen overlapping the position of a microtubule. However, considering the dense microtubule cytoskeleton in these cells faithful analysis of co-localization was impaired. From these results, it is evident that GFPClasp2 changes its localization in response to Met activation, suggesting that it is regulated by Met signaling. However, the precise nature of this relocalization awaits closer analysis. Brady Eason Page 8 B1-3_1 min B1-3_0 min B1-3_5 min Figure 8: Immunoflourescence images with GFP-Gab1 depicted in green, nuclei in blue, and microtubules in red. Used as a positive control showing that GFP-Gab1 relocalizes to the cortex of the MDCK cells upon Met stimulation as expected. Bottom row images correspond to top row images with microtubules shown. FS_Clasp2_0 min FS_Clasp2_2 min FS_Clasp2_5min FS_CLASP2_5 min Figure 9: Immunoflourescence images with GFP-Clasp2 depicted in green, nuclei in blue, and microtubules in red. No HGF stimulation, 2 minutes and 5 post stimulation images are shown. Bottom row corresponds to the top row with microtubule signal overlay. Brady Eason Page 9 DISCUSSION Optimizing both expression and detection of GFP-Clasp2 in HeLa 299s is important for many future experiments in the laboratory, even if the co-immunoprecipitation of GFP-Clasp2 with HA-Gab1 in the optimized conditions did not support the association seen in the previous experiments. As alluded to earlier, this could be a result of the cell type used (i.e. that a bridging protein present in HEK 293s is absent in HeLa cells or that the optimized protein levels in HeLa 299s are still not sufficient). In future experiments other cell types such as COS cells that have been shown in the literature to be quite successful when used in GFP-Clasp1/2 experiments [5] could be procured and used alternatively. The movies and immunoflourescence experiments strongly suggest that GFP-Clasp2 localization is dependent on Met signaling. In later experiments, instead of imaging with MDCK cells, which as epithelial cells adopt a cylindrical shape, a flatter cell type, such as the abovementioned COS cells, may be beneficial. In these cells the microtubule lattice will arrange itself in fewer focal planes, allowing for a greater overall view of the network. In order to improve studies of GFP-Clasp1/2 localizing to the microtubule network, it is recommended that livecell imaging of GFP-Clasp1/2 together with a mCherry-tubulin construct be transfected into the MDCK cells, which stably express GFP-Clasp1/2. Our experiments suggest that the localization of Clasp protein is regulated by Met signaling. In future experiments we will determine if the association of Gab1 with Clasp is required for this re-localization. To address this, we will compare the HGF/Met-dependent localization of Clasp1/2 in wild-type mouse embryonic fibroblasts (MEFs) and MEFs from GAB1-deficient mice. If Clasp protein re-localizes in response to HGF in wild-type, but not in GAB1-deficient fibroblast, this would indicate that Clasp is recruited via Gab1 to its new locations during cell migration and may hence indicate a potential function of the Gab1-Clasp interaction. Brady Eason Page 10 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. Birchmeier, C., et al., Met, metastasis, motility and more. Nat Rev Mol Cell Biol, 2003. 4(12): p. 915-25. Sachs, M., et al., Essential role of Gab1 for signaling by the c-Met receptor in vivo. J Cell Biol, 2000. 150(6): p. 1375-84. Drabek, K., et al., Role of CLASP2 in microtubule stabilization and the regulation of persistent motility. Curr Biol, 2006. 16(22): p. 2259-64. Mimori-Kiyosue, Y., et al., CLASP1 and CLASP2 bind to EB1 and regulate microtubule plus-end dynamics at the cell cortex. J Cell Biol, 2005. 168(1): p. 141-53. Akhmanova, A., et al., Clasps are CLIP-115 and -170 associating proteins involved in the regional regulation of microtubule dynamics in motile fibroblasts. Cell, 2001. 104(6): p. 923-35. Maiato, H., et al., Human CLASP1 is an outer kinetochore component that regulates spindle microtubule dynamics. Cell, 2003. 113(7): p. 891-904. Pereira, A.L., et al., Mammalian CLASP1 and CLASP2 cooperate to ensure mitotic fidelity by regulating spindle and kinetochore function. Mol Biol Cell, 2006. 17(10): p. 4526-42. Tai, A.L., et al., High-throughput loss-of-heterozygosity study of chromosome 3p in lung cancer using single-nucleotide polymorphism markers. Cancer Res, 2006. 66(8): p. 4133-8. Maitra, A., et al., High-Resolution Chromosome 3p Allelotyping of Breast Carcinomas and Precursor Lesions Demonstrates Frequent Loss of Heterozygosity and a Discontinuous Pattern of Allele Loss. Am J Pathol, 2001. 159(1): p. 119-130. Ghoussoub, R.A., et al., Expression of c-met is a strong independent prognostic factor in breast carcinoma. Cancer, 1998. 82(8): p. 1513-20. Camp, R.L., E.B. Rimm, and D.L. Rimm, Met expression is associated with poor outcome in patients with axillary lymph node negative breast carcinoma. Cancer, 1999. 86(11): p. 2259-65. Cappuzzo, F., et al., MET increased gene copy number and primary resistance to gefitinib therapy in non-small-cell lung cancer patients. Ann Oncol, 2009. 20(2): p. 298-304. Brady Eason Page 11