New method implementation checklist QPCMI12001
advertisement

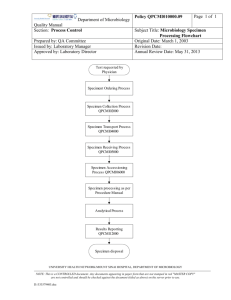
Department of Microbiology Quality Manual Section: Process Control Prepared by: QA Committee Issued by: Laboratory Manager Approved by: Laboratory Director Form QPCMI12001.09 Page 1 of 3 Subject Title: New Method/Test/Project/Change Implementation Checklist Form Original Date: July 22, 2003 Revision Date: April 20, 2007 Annual Review Date: May 31, 2013 Purpose: This checklist provides a guide to identify items to be considered before implementation of any change or new method, test or new project. Procedure: 1. New method/project/test has to be previously approved in principle at the Senior Staff Meeting. 2. Complete this form prior to implementation of a new test/methodology/project/change to identify action items 3. Present the completed form at the Departmental Operations Meeting. Name of New Method/Test/Project or Change: _______________________________________ Date: _____________ Approximate volume (if applicable): _______________ Requested by: _________________ Originated from (Service/Client):__________________ Approved by: ______________________ Approved date: ________________________ Project Leader: _____________________ Item Consultation with stakeholders Specimen Transportation requirement Specimen Management requirement Workflow issues Space/Bench requirement Action needed: Responsible by: Status Human Resources requirement: - hire - Labour/management negotiation? UNIVERSITY HEALTH NETWORK/MOUNT SINAI HOSPITAL, DEPARTMENT OF MICROBIOLOGY NOTE: This is a CONTROLLED document. Any documents appearing in paper form that are not stamped in red "MASTER COPY" are not controlled and should be checked against the document (titled as above) on the server prior to use. D:\533563025.doc Department of Microbiology Quality Manual Section: Process Control Item Supply/Equipment requirement Financial requirement Billing procedure LIS requirement: - Interface to HIS Cerner Misys Meditech Bacrest Meditech RVH - HIS setup - interface to instrument - test setup - report setup - workload setup - Softstore setup Changes to Procedure Manual Training requirement Communication about implemented change: - with clients - with other department(s) - with staff - with specimen management at: UHN MSH 6th floor CHC Ajax/Pickering Bridgepoint - with call centre - update web site - SOP - lab dictionary For new test, send application/notification to: Ontario Laboratory Licence QMP-LS Ontario Lab Accreditation College of Pathologists Form QPCMI12001.09 Page 2 of 3 Subject Title: New Method/Test/Project/Change Implementation Checklist Form Action needed: Responsible by: Status UNIVERSITY HEALTH NETWORK/MOUNT SINAI HOSPITAL, DEPARTMENT OF MICROBIOLOGY NOTE: This is a CONTROLLED document. Any documents appearing in paper form that are not stamped in red "MASTER COPY" are not controlled and should be checked against the document (titled as above) on the server prior to use. D:\533563025.doc Department of Microbiology Quality Manual Section: Process Control Item Form QPCMI12001.09 Page 3 of 3 Subject Title: New Method/Test/Project/Change Implementation Checklist Form Action needed: Responsible by: Status Post implementation audit(s) UNIVERSITY HEALTH NETWORK/MOUNT SINAI HOSPITAL, DEPARTMENT OF MICROBIOLOGY NOTE: This is a CONTROLLED document. Any documents appearing in paper form that are not stamped in red "MASTER COPY" are not controlled and should be checked against the document (titled as above) on the server prior to use. D:\533563025.doc