ELC Science Worksheet
Component 3: Materials from the Earth
Component 3: Materials from the Earth
Learning Outcome 1
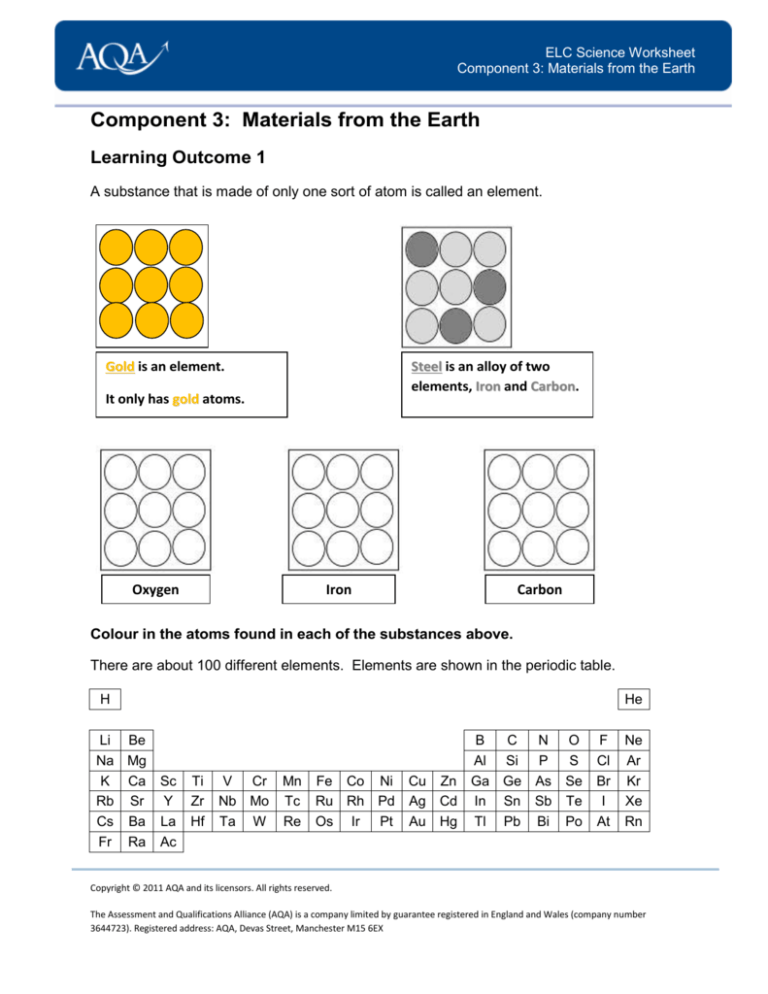
A substance that is made of only one sort of atom is called an element.
Gold is an element.
Steel is an alloy of two
elements, Iron and Carbon.
It only has gold atoms.
Oxygen
Iron
Carbon
Colour in the atoms found in each of the substances above.
There are about 100 different elements. Elements are shown in the periodic table.
H
Li Be
Na Mg
K Ca Sc
Rb Sr Y
Cs Ba La
Fr Ra Ac
He
Ti
Zr
Hf
B
C
N O
Al Si P
S
V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se
Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te
Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po
F
Cl
Br
I
At
Ne
Ar
Kr
Xe
Rn
Copyright © 2011 AQA and its licensors. All rights reserved.
The Assessment and Qualifications Alliance (AQA) is a company limited by guarantee registered in England and Wales (company number
3644723). Registered address: AQA, Devas Street, Manchester M15 6EX
ELC Science Worksheet
Component 3: Materials from the Earth
Your teacher will tell you which elements are metals. Shade in the elements that
are metals.
Use the words in the table below to complete the blanks in these sentences.
one thousand
one
atoms
compounds
table
element
one hundred
metals
non-metals
All substances are made up of ___________________________ .
A substance that is made up of only _______ type of atom is called an _______________.
There are about __________________ different elements.
Elements are shown in the Periodic ________________________ .
Most of the elements in the periodic table are ________________ .
Copyright © 2011 AQA and its licensors. All rights reserved.
The Assessment and Qualifications Alliance (AQA) is a company limited by guarantee registered in England and Wales (company number
3644723). Registered address: AQA, Devas Street, Manchester M15 6EX
ELC Science Worksheet
Component 3: Materials from the Earth
Component 3: Materials from the Earth
Learning Outcome 2
Elements
A substance that is made of only one sort of atom is called an element. All the elements
can be found in the periodic table. For example ‘gold’ is made of only gold atoms; carbon is
only made from carbon atoms; and oxygen is only made from oxygen atoms.
Compounds
A compound is a substance that is made of two or more different elements that are
chemically joined together. For example:
carbon
+
oxygen
=
carbon
dioxide
In the table below write C if it is a compound and E if it is an element.
(Clue: If you can’t find it on the periodic table it must be a compound)
Substance
C or
E?
Substance
Water
Tin
Sodium
Gold
Iron
Carbon
dioxide
Salt
Copper oxide
Copper
Calcium
Calcium
carbonate
Hydrogen
C or
E?
Sulfur dioxide
Oxygen
Copyright © 2011 AQA and its licensors. All rights reserved.
The Assessment and Qualifications Alliance (AQA) is a company limited by guarantee registered in England and Wales (company number
3644723). Registered address: AQA, Devas Street, Manchester M15 6EX
ELC Science Worksheet
Component 3: Materials from the Earth
Chemical reactions can be represented by word equations.
Chemists sometimes write word equations to show what happens in a chemical reaction.
Try to complete the word equations below (use the words in the table to help you):
oxide
copper
sodium
chlorine
calcium
oxygen
sulfur
carbon
Hydrogen + O_______________ water
Copper + O___________ C___________ oxide
Sodium + C_______________ S____________ chloride
Sulfur + Oxygen S_______________ dioxide
Copyright © 2011 AQA and its licensors. All rights reserved.
The Assessment and Qualifications Alliance (AQA) is a company limited by guarantee registered in England and Wales (company number
3644723). Registered address: AQA, Devas Street, Manchester M15 6EX
ELC Science Worksheet
Component 3: Materials from the Earth
Component 3: Materials from the Earth
Learning Outcome 3
Complete the blank spaces in the sentences about limestone.
Limestone rock contains at least 50% calcium c_____________.
Limestone is a rock that is q__________. Limestone rock is used as a b_________
material. The rock can be cut into b________ and used in buildings. Limestone rock
can also be shaped or sculptured. Lots of older buildings have examples of these
sculptures.
Copyright © 2011 AQA and its licensors. All rights reserved.
The Assessment and Qualifications Alliance (AQA) is a company limited by guarantee registered in England and Wales (company number
3644723). Registered address: AQA, Devas Street, Manchester M15 6EX
ELC Science Worksheet
Component 3: Materials from the Earth
Match each picture to its description.
A
1
Limestone is quarried and
transported to be cut in to
different sizes.
B
2
Limestone is cut in to
different size. Here a rough
piece of limestone is being
cut into a finished block.
C
3
The finished blocks are
stored ready to be
transported to building
suppliers.
Copyright © 2011 AQA and its licensors. All rights reserved.
The Assessment and Qualifications Alliance (AQA) is a company limited by guarantee registered in England and Wales (company number
3644723). Registered address: AQA, Devas Street, Manchester M15 6EX
ELC Science Worksheet
Component 3: Materials from the Earth
D
4
The blocks are used to for
buildings. Some limestone
blocks are sculptured in to
ornate shapes. Can you spot
the gargoyle in this picture?
Copyright © 2011 AQA and its licensors. All rights reserved.
The Assessment and Qualifications Alliance (AQA) is a company limited by guarantee registered in England and Wales (company number
3644723). Registered address: AQA, Devas Street, Manchester M15 6EX
ELC Science Worksheet
Component 3: Materials from the Earth
Component 3: Materials from the Earth
Learning Outcome 4
Metal ores
Complete the sentences.
Metals are found in r__________________ in the Earth’s crust.
A few metals like g____________ and copper occur as free metal, un-combined.
These can withstand the action of air and w____________ without being converted into
c____________.
Reactive metals can be obtained from ores
Most metals have to be extracted from compounds in ores.
Some metals are found in the natural state in the ground.
Put a tick by the metals in the list below that exist in their natural state.
Metal
Do they exist in their natural state
in the ground?
Aluminium
Iron
Gold
Zinc
Lead
Silver
Copyright © 2011 AQA and its licensors. All rights reserved.
The Assessment and Qualifications Alliance (AQA) is a company limited by guarantee registered in England and Wales (company number
3644723). Registered address: AQA, Devas Street, Manchester M15 6EX
ELC Science Worksheet
Component 3: Materials from the Earth
Recycling metals
Metals can be recycled without losing any of their properties.
The table below shows some different types of waste that a recycling centre dealt with
during one year.
Use the table to draw a bar chart to show how much of each type of waste is recycled.
Type of waste
%
recycled
Garden waste
21
Paper and board
18
Kitchen waste
17
Glass
7
Scrap metals
5
The sentences below are statements about the effect of recycling metals.
Use words from the box to complete the sentences.
age
ores
damages
recycled
energy
reduces
local money
waste
Recycling ____________ the amount of energy needed.
Recycling means less mining that _______________ the environment.
Recycling metals uses less energy than extracting them from _________ .
Recycling of aluminium cans eliminates ____________, saves ___________,
conserves natural resources, reduces the use of landfill sites and provides
_____________ for charities and _____________ government.
A number of factors affect the amount of waste that is _____________ .
Household size and the average _________ of residents have significant effects
Copyright © 2011 AQA and its licensors. All rights reserved.
The Assessment and Qualifications Alliance (AQA) is a company limited by guarantee registered in England and Wales (company number
3644723). Registered address: AQA, Devas Street, Manchester M15 6EX
ELC Science Worksheet
Component 3: Materials from the Earth
on recycling.
Component 3: Materials from the Earth
Learning Outcome 5
Iron is converted into steel
Complete the sentences.
Cast iron contains c________ that makes it brittle. Steel is made by reducing the
c_______________ content to less than 1 %
Steel is an alloy. An alloy is a mixture that contains at least
one m_______________ .
Pure Iron and Cast Iron
Pure iron is too soft to use as a pure metal so it is alloyed with other elements to make
it stronger.
Cast iron is an alloy of iron (96%) and carbon and silicon. Cast iron is hard, strong and
doesn’t corrode (rust), however it is brittle. Objects made of cast iron include door
stops, drain covers, stairs and handrails, and plaques.
Copyright © 2011 AQA and its licensors. All rights reserved.
The Assessment and Qualifications Alliance (AQA) is a company limited by guarantee registered in England and Wales (company number
3644723). Registered address: AQA, Devas Street, Manchester M15 6EX
ELC Science Worksheet
Component 3: Materials from the Earth
Match each type of steel to its description
Type of steel
Mild steel (less than 0.25 % carbon)
Medium steel (0.25-0.45 % carbon)
High carbon steel (0.45-1.5 % carbon)
Cast iron (2.5-4.5 % carbon)
Description
Easily moulded into complicated
shapes
Hard and brittle
Pliable, can be bent without
breaking
Tougher than mild steel, more
springy
Copyright © 2011 AQA and its licensors. All rights reserved.
The Assessment and Qualifications Alliance (AQA) is a company limited by guarantee registered in England and Wales (company number
3644723). Registered address: AQA, Devas Street, Manchester M15 6EX
ELC Science Worksheet
Component 3: Materials from the Earth
Component 3: Materials from the Earth
Learning Outcome 6
Copper coins
Copper is too soft to use as a pure metal so it is alloyed with other elements to make it
stronger. Most British coins are made from copper that has been alloyed with other
metals.
Copper coins are actually an alloy of three metals. 97% copper,
2.5% zinc and 0.5% tin; therefore copper coins should really be
called bronze!
Silver coins are not made from the element silver. They are an
alloy of copper (75%) and nickel (25%)
The one pound coin is composed of 70% copper, 5.5% nickel and
24.5% zinc, making this a bronze coin.
Copyright © 2011 AQA and its licensors. All rights reserved.
The Assessment and Qualifications Alliance (AQA) is a company limited by guarantee registered in England and Wales (company number
3644723). Registered address: AQA, Devas Street, Manchester M15 6EX
ELC Science Worksheet
Component 3: Materials from the Earth
Gold
Gold is too soft and too expensive for many people. It is usually made into a gold alloy.
The amount of gold in the alloy is measured in carats. Pure gold is 24 carat.
Match the gold carat alloy with the correct property.
Carat
Property
18
This is the cheapest alloy
because it has the least amount
gold (9/24 or 37.5% gold and
62,5% copper). This alloy is
much harder than the others.
9
This is the most popular alloy for
jewellery because it is not too
expensive. It contains 18/24 or
75% gold and 25% copper.
22
This is the most expensive alloy
because it contains the most gold
(91.6% gold and 8.4% copper).
This is the softest alloy.
Copyright © 2011 AQA and its licensors. All rights reserved.
The Assessment and Qualifications Alliance (AQA) is a company limited by guarantee registered in England and Wales (company number
3644723). Registered address: AQA, Devas Street, Manchester M15 6EX
ELC Science Worksheet
Component 3: Materials from the Earth
Which metal is being described below?
Draw a ring around the correct answer.
This metal is too soft and expensive so it is alloyed with copper.
Iron
copper
gold.
‘Carat’ is used to describe the purity of this metal
Iron
gold.
Coins are made from an alloy of this metal
Iron copper
gold.
This metal is alloyed with carbon to make it hard, strong and resistant to
corrosion
Iron
copper
copper
gold.
This is a pure metal that reacts with oxygen and rusts
Iron
copper
gold.
Copyright © 2011 AQA and its licensors. All rights reserved.
The Assessment and Qualifications Alliance (AQA) is a company limited by guarantee registered in England and Wales (company number
3644723). Registered address: AQA, Devas Street, Manchester M15 6EX
ELC Science Worksheet
Component 3: Materials from the Earth
Component 3: Materials from the Earth
Learning Outcome 7
Properties of copper and its uses
Complete the sentences.
Copper is a metal that is easy to w_________
It is a good conductor of e______________
It is a good t____________ conductor
It has a high m_______________ point and b_____________ point
Un-reactive m____________ .
It is used for e________________ wiring.
It is used for making water p___________ because it is quite easy to b__________ and
does not r_____________ .
Aluminium is a useful metal
Density is a measure of how heavy something is for its size.
Aluminium is less dense than iron. If you had a block of iron and a block of aluminium
the same size, which one would weigh more?
The ___________________ block would weigh more.
Aluminium is very r__________ to corrosion.
Copyright © 2011 AQA and its licensors. All rights reserved.
The Assessment and Qualifications Alliance (AQA) is a company limited by guarantee registered in England and Wales (company number
3644723). Registered address: AQA, Devas Street, Manchester M15 6EX
ELC Science Worksheet
Component 3: Materials from the Earth
Uses of aluminium
Aluminium can be used to make many things.
Some of these are listed below.
Complete each item by filling in the letters in the spaces.
Stepl_dd_rs
A_r_ra_t
C_rs
G r _ _ n h _ _ s _e s
W_n_owfr_m_s
M_lkb_tt_et_ps
Bak_ngtr_ys
Copyright © 2011 AQA and its licensors. All rights reserved.
The Assessment and Qualifications Alliance (AQA) is a company limited by guarantee registered in England and Wales (company number
3644723). Registered address: AQA, Devas Street, Manchester M15 6EX
ELC Science Worksheet
Component 3: Materials from the Earth
Component 3: Materials from the Earth
Learning Outcome 8
Complete the statements by choosing the correct word from the brackets.
Crude oil is a mixture of compounds that (can / cannot) separated.
Oil and gas were formed from the remains of plants and animals
over (millions / hundreds) of years.
When dead matter decays, it is turned by bacteria in the
(absence / presence) of oxygen into crude oil.
As the mud layers built up, high temperature and pressure was created.
This converted the material (slowly / quickly) into oil and gas.
Earth movements caused the rock to split and the oil and gas was trapped
beneath the layers of (porous / non-porous) rocks.
Gas and oil move upwards through (porous / non-porous) rock.
Oil (fields/wells) can be found under the North Sea.
Copyright © 2011 AQA and its licensors. All rights reserved.
The Assessment and Qualifications Alliance (AQA) is a company limited by guarantee registered in England and Wales (company number
3644723). Registered address: AQA, Devas Street, Manchester M15 6EX
ELC Science Worksheet
Component 3: Materials from the Earth
Crude oil is a mixture, made up of different compounds with different boiling points.
The table below shows the steps in refining crude oil. Put the steps in the correct order from 1
to 7. The first one has been done for you.
Letter
Statements
A
The liquid evaporates and the vapour
condenses at different temperatures.
B
The fraction with the lowest boiling point
comes at the top of the tower.
C
Crude oil is heated in a fractionating tower.
D
The fraction with the highest boiling point
comes at the bottom of the tower.
E
The light fractions, which are usually liquid,
have lower boiling points and come out first.
F
Crude oil is extracted from oil fields below
the ground.
G
This process is used to produce a range of
useful oils and fuels.
Correct order
1
Copyright © 2011 AQA and its licensors. All rights reserved.
The Assessment and Qualifications Alliance (AQA) is a company limited by guarantee registered in England and Wales (company number
3644723). Registered address: AQA, Devas Street, Manchester M15 6EX
ELC Science Worksheet
Component 3: Materials from the Earth
Component 3: Materials from the Earth
Learning Outcome 9
Burning fuels release gases
Use the words given in the box to complete the statements.
energy
dioxide
monoxide
power
sulfur
vapour
Fuels are burnt in ____________ stations, factories and vehicle engines.
Fuels are hydrocarbons that release ______________ when burnt.
The products formed when a hydrocarbon burns are water _____________, carbon
dioxide and sometimes carbon ___________ .
Coal contains ______________ as impurities.
When an impure coal is burnt it releases sulfur __________________ .
Copyright © 2011 AQA and its licensors. All rights reserved.
The Assessment and Qualifications Alliance (AQA) is a company limited by guarantee registered in England and Wales (company number
3644723). Registered address: AQA, Devas Street, Manchester M15 6EX
ELC Science Worksheet
Component 3: Materials from the Earth
The impact on the environment of burning fossil fuels:
Use the words given in the box to complete the statements.
acidic
acid rain
animals
dioxide
killed
limestone
country
The effects of acid rain:
The two gases responsible for ______ ________ are sulfur dioxide and nitrogen dioxide.
Acid rain eats away buildings made of ______________, concrete and metal.
It causes lakes to become ___________ and as a result of that many plants and
______________die. Forests may be destroyed in one ____________ as the effect of
sulfur ________________ may be felt far away.
Fish can be ___________ in a lake by the burning of fossil fuels in a country far away.
Use the words given in the box to complete the statements.
carbon dioxide
dry
heat
hot
lands melt
rise
starve
weather
The Greenhouse effect (global warming effect):
The main cause is ____________________, which warms the Earth by trapping
_____________ . Polar ice caps __________ in the Arctic and Antarctica.
Sea levels may ____________ . More frequent, unpredictable _____________
patterns occur. Low-lying ____________ will disappear.
Some agricultural regions will become too _________ and _________ to grow crops.
There will be famine and people will __________ .
Copyright © 2011 AQA and its licensors. All rights reserved.
The Assessment and Qualifications Alliance (AQA) is a company limited by guarantee registered in England and Wales (company number
3644723). Registered address: AQA, Devas Street, Manchester M15 6EX
ELC Science Worksheet
Component 3: Materials from the Earth
Component 3: Materials from the Earth
Learning Outcome 10
The poisonous gas carbon monoxide is produced when fuels burn in a limited
supply of air
When a fuel burns in a limited supply of air, the poisonous gas produced is carbon
monoxide.
This is called incomplete c___________________ .
In a chemical reaction, two or more reactants combine to form products.
A
+
B
C
(reactants)
+
D
(products)
Chemical reactions can be represented by word equations.
Carbon
+
oxygen
carbon monoxide
What happens when fuels are burnt?
The gases released into the atmosphere when a fuel burns may include carbon dioxide,
water (vapour), carbon monoxide and sulphur dioxide.
Burning oil
(or their
fractions
e.g. petrol)
carbon dioxide
Poisonous
gases given
off
carbon monoxide
sulphur dioxide
soot (particulates)
Copyright © 2011 AQA and its licensors. All rights reserved.
The Assessment and Qualifications Alliance (AQA) is a company limited by guarantee registered in England and Wales (company number
3644723). Registered address: AQA, Devas Street, Manchester M15 6EX
ELC Science Worksheet
Component 3: Materials from the Earth
How are carbon dioxide and carbon monoxide produced?
The poisonous gas carbon monoxide is produced when fuels burn in a limited supply of air.
Fuel
+
+
Fuel+
+
+
+
Lots
of air
Little
air
=
=
Carbon
dioxide
Carbon
monoxide
Complete the following sentences by replacing the missing words below.
combustion
fires
limited
monoxide
poisonous
reactant
When fuels are burnt in a l_______________ supply of oxygen, incomplete combustion occurs.
This produces carbon m________________.
This is an odourless (can’t smell it) p________________ gas.
Plenty of ventilation is needed when gas f________________ and coal fires are used.
Copyright © 2011 AQA and its licensors. All rights reserved.
The Assessment and Qualifications Alliance (AQA) is a company limited by guarantee registered in England and Wales (company number
3644723). Registered address: AQA, Devas Street, Manchester M15 6EX