Spider Silk Final Project
advertisement

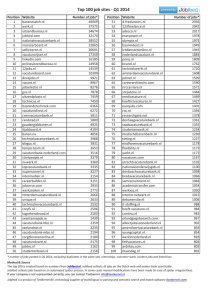
Chris Kao Greg Stahr Erik Thiede Liz Moser Final Model Project Write-Up Spider silk has garnered much interest in fields such as industry, military defense, and structural materials due to its combination of high strength, ductility, and light weight. Many scientists around the world are studying the properties of this amazing material to find ways to mass produce silk, or create artificial material with similar properties. The most studied material is the dragline silk of the Golden-orb weaving spider. Understanding the structure of spider silk will help give insight on this versatile material. Spider silk is composed of two proteins, spidroin 1 and spidroin 2. These spidroin proteins contain a significant amount of amino acids glycine and alanine, at 42% and 25% respectively. The reason glycine and alanine are so abundant is that they do not contain bulky side groups, making them easier to pack tightly together and form crystalline regions. The lack of bulky side groups also helps the protein gain flexibility and elasticity. Amino acids glutamine, serine, leucine, valine, proline, tyrosine and arginine make up the rest of the protein composition, with spidroin 1 and 2 differing mostly in their proline and tyrosine content. Spidroin has three main structural amino components: polyalanine chains (4-9 alanines linked together), glycine rich repeated regions of five amino acids, and glycine rich repeating segments of three amino acids. Two antiparallel polyalanine chains are connected by hydrogen bonds to form beta sheets. These beta sheets help give solid crystalline structure and high tensile strength. The segments of five amino acids have 180 degree turns after each repeat to form a bspiral, and the segments of three amino acids serve as an intermediate between the beta sheets and b-spirals. The combination of the ordered beta sheets and amorphous glycine rich spirals regions give spider silk its high tensile strength, toughness, and extensibility. The main reason Spider Silk is such an interesting compound is it’s stress-strain potential. The stress strain equation relates the stress (force over area, σ) exerted on an object in comparison to the deformation felt by it, its strain (ε). These two quantities are linked by the stress strain equation, σ=Eε, a more general formation of Hooke’s law (F=kx). E is the modulus which relates the two quantities. Depending on the type of spider silk, it has both very high tensile stress and tensile strain capabilities. For tensile calculations, these two properties are related by the Young’s modulus. Dragline silk has an initial value for the Young’s modulus of 10GPa (Gosline et al, Mechanical design) very high for an elastomeric protein. It can also accept incredible amounts of stress, into the gigapascal range (it is worth noting that the modulus and the max amount of stress have the same units, this is because strain is unitless). Viscid spider silk, in turn, can undergo incredible amounts of strain without breaking. It can deform almost a third as much as natural rubber, while still maintaining ten times the strength (Gosline, Mechanical Design). The second important property about spider silk is its hysteresity. For an ideal spring, the energy of the spring is a state function, it is only dependent on the deformation of the spring. However, due to the laws of thermodynamics, some energy must be lost for any deformation of a real spring. This loss in energy results in a corresponding loss in the restorative force of a spring or elastomer. (A decrease in potential energy leads to the elastomer having less ability to do work, in turn leading to a lower force exerted as W=F*dx). While this is a bit of a disadvantage for dragline Spider silk, it allows viscid silk to be energy dissipating. However, it is worth noting that, as a consequence, spider silk only has a limited number of useful deformations. Figure 1: Curves showing the hysteresis of both dragline and viscid spider silk (Gosline et. al, Elastic Proteins) Figure 2: Stress-strain curves for both viscid and dragline spider silk along with other elastomers. (Gosline et al, Elastic Proteins) In 2009, spider silk was used as thread to weave an elaborate golden cloth in Madagascar. While the cloth is beautiful, it took over four years to extract the silk from over one million golden orb spiders. This exemplifies the main reason more progress with spider silk has been made. It takes an incredible amount of time and manpower to extract and process significant amounts of the durable silk, making it inefficient in terms of cost. However, using spider silk as a fabric is very simple. Scientists, on the other hand, have still expressed an interest in spider silk due to its incredible toughness. Spider silk exhibits incredible extensibility and strength that make it incredibly tough; in fact, it is significantly tougher than both Kevlar and steel. Spider silk has been discussed as an alternative to Kevlar body armor. It is much tougher, and therefore would be able to withstand a bullet’s force much more effectively (due largely to its extensibility). Spider silk has also been suggested as a material for structural reinforcement. It has been speculated that the silk fibers could be used to reinforce bridges and elevator ropes. Aside from this, there has been much discussion about spider silk in biomedical implants. Spider silk maintains its toughness and strength even when exposed to high temperatures and water. Therefore it is an ideal candidate for reinforcing bones, and replacing other damaged tissues such as skin and even heart valves. Lastly, spider silk has a very low glass transition temperature (around -60C) — the temperature at which a liquid turns into a glass, but not a crystallized solid — which allows it to stay tough at a low temperature making it a suitable material for parachutes. All of these applications are able to take advantage of the incredible strength, toughness, and extensibility spider silk exhibits. In this model, we used foam rectangles as representations of beta sheets and pipe cleaners as representations of the cross-links and amorphous regions between them. The pleated beta sheets that represent the secondary structure of the protein in dragline spider silk consist of polyalanine regions connected by hydrogen bonds. Amorphous regions order themselves in the cross-links between the beta sheets and add to the system’s elasticity. These consist of glycine-rich spirals, which are represented by the pipe Cross-links cleaners. Beta sheets Amorphous regions Our protein consists of the amino acids alanine, glycine, glutamine. leucine, tyrosine, and serine. The amino acid sequence is: AGQGGYGGLGSQG These specific amino acids do not have bulky side chains, enabling the protein to coil tightly and adds to its elasticity and flexibility. This protein is also very hydrophobic and solidifies by losing water. http://www.absoluteastronomy.com/topics/Spider_silk http://www.tx.ncsu.edu/jtatm/volume5issue1/Articles/Saravanan/Saravanan _Full_170_05.pdf http://www.microlabnw.com/index/gallery/SpiderSilk.html http://www.wired.com/wiredscience/2009/09/spider-silk/ http://www.scientificamerican.com/article.cfm?id=new-nanomaterialfuses-sp The Mechanical Design of Spider Silks, Gosling et al. JExpBiol, 1999. Elastic proteins: biological roles and mechanical properties, John Gosline, Margo Lillie, Emily Carrington, Paul Guerette, Christine Ortlepp, and Ken Savage Comparative structures and properties of elastic proteins, Arthur S. Tatham and Peter R. Shewry