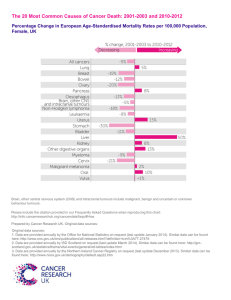
A NEW LOOK ON INTESTINAL SMOOTH MUSCLE TUMORS
advertisement

A NEW LOOK ON INTESTINAL SMOOTH MUSCLE TUMOURS: CLINICAL, HISTOLOGIC AND IMMUNOHISTOCHEMICAL ASPECTS FOR RECLASSIFICATION C.P.H.J. Maas, E-mail: cerielmaas@yahoo.com According to the histological classification of tumours of the intestines of domestic animals published by the World Health Organization, primary tumours of the intestinal tract can be of epithelial, neuroendocrine, haematopoietic or mesenchymal origin (1). Most gastrointestinal (GI) mesenchymal tumours with smooth muscle appearance have been diagnosed as either leiomyoma (LM) or leiomyosarcoma (LMS) based on light microscopic examination of haematoxylin and eosin (HE) stained tissue sections (2). Histological differentiation between benign and malignant tumours is carried out by scoring tissue differentiation, nuclear pleomorphism, cellularity, presence of nucleoli, mitotic count, and amount of necrosis, bleeding and inflammation present in the tissue section (3,4). However, if these tumours are evaluated using immunohistochemical (IH) analysis they actually prove to be a heterogeneous group of mesenchymal tumours of which some fail to express smooth muscle markers (1,5), which may suggest another origin than smooth muscle tissue. IH and ultrastructural studies of these tumours in human and veterinary patients show differentiation towards either smooth muscle (myogenic), or neural tissue (neurogenic), a combination of both (mixed), or undifferentiated tissue (anaplastic) (1,2,6). IH stains enable differentiation between tumours from smooth muscle origin and stromal tumours. In the human and veterinary literature, various IH stains are reported: Smooth muscle actin (SMA) and desmin are used as markers for myogenic differentiation; S-100 is applied as a marker of neurogenic differentiation; vimentin is reported as an indicator of mesenchymal origin of tissue and KIT (CD 117) is used as a marker of a receptor tyrosine kinase. This receptor tyrosine kinase can be found in several tissues including hematopoietic progenitor cells, mast cells, melanocytes and the interstitial cells of Cajal (ICC). These ICC form a network in the GI tract that coordinates GI peristalsis (2,7,8). In humans, many of these tumours were formerly diagnosed as smooth muscle tumours but have been reclassified as gastrointestinal stromal tumours (GIST), which in human medicine are defined as KIT expressing tumours. Uncontrolled activity of KIT is an important step in the development of GIST (1,7). Apart from the primary surgical therapy in humans, an additional medical treatment under investigation is selective receptor tyrosine kinase inhibitor (imatinib mesylate) therapy (8). In veterinary medicine, a higher incidence of malignant tumours with smooth muscle appearance is reported in the small intestine and caecum, in contrast to benign tumours which were located more often in the stomach (2,5,7). A retrospective study (9) was performed in canine patients, which had been diagnosed with either LM or LMS of the small intestine and caecum, by means of histological evaluation of paraffin-embedded and HE-stained tissue sections. Clinical data (patient signalment; medical history; clinical, diagnostic imaging and surgical findings; survival data) were reviewed, from medical records and telephone interviews with the veterinarian or owner. All HE slides were (re)evaluated and scored. The original tissue sections were all investigated for expression of SMA, desmin, S-100, vimentin and KIT. The following method for (re)classification of intestinal tumours with light microscopic appearance of smooth muscle was used (also see the flow-chart): - LM are histological benign, express either or both SMA and desmin, with or without expression of vimentin, and do not express S-100 and KIT. If the tumour should express KIT and vimentin, or S100 and vimentin it is reclassified, respectively, as GIST or GIST-like, regardless of histological appearance. - LMS are comparable to LM, but these tumours show a malignant appearance on histology. - GIST express KIT and vimentin, either with or without expression of SMA, desmin and S-100. They can show both a benign or malignant histological appearance. - GIST-like tumours are histologically and IH identical to GIST but lack expression of KIT. In this study (9) of which only part of the data is reported here, 47 dogs were included with small intestinal tumours. Of the 40 dogs with original diagnosis of LMS, one was reclassified as LM, 17 were reclassified as GIST and 13 as GIST-like; of the seven dogs originally diagnosed with LM, two were reclassified as GIST and four as GIST-like. In 25 patients, the tumour was located in the caecum. Of the 23 tumours originally diagnosed as LMS, 22 were reclassified as GIST and one as GIST-like. The two tumours originally diagnosed as LM were reclassified as one GIST and one GIST-like tumour. Mean age for dogs with small intestinal tumours (9.1± 2.3 years) was lower than for dogs with caecal tumours (10.7± 1.9 years). In contrast to dogs with intestinal tumours which all showed clinical signs, four dogs with caecal tumours showed no clinical signs at all and the mass was an incidental finding. In dogs with intestinal tumours, weight loss was observed more often, whereas intestinal perforation and peritonitis were observed more commonly in dogs with caecal tumours. In general the caecal tumours showed higher scores for necrosis and bleeding. Between the different reclassified tumours, the small intestinal LMS showed most nuclear pleomorphism, poorest differentiation, most mitosis and nucleoli. Of the investigated variables, an increased tumour diameter was observed an important negative prognostic indicator and castrated or spayed dogs were observed to have better survival statistics than intact dogs. For the 42 dogs with small intestinal tumours that were surgically-treated, the overall 1- and 2-year survival time was 63% and 52% whereas, for the 19 dogs with caecal tumours that were surgically treated, this was 84% and 66% respectively. Of the surgically-treated dogs, nine with a small intestinal tumour and one with a caecal tumour, died within 15 days because of surgery or tumourrelated complications. For the remaining dogs with small intestinal tumours, the 1- and 2-year recurrence free period was 80% and 67% whereas, for the dogs with the caecal tumours, this was 83% and 62% respectively. IH analysis showed that many GIST (1,2,7) and GIST-like tumours (9) can display differentiation in mixed myogenic and neurogenic direction, which is most suggestive of a relationship with primitive mesenchymal cells capable of pluripotential differentiation (1). The ICC are in close contact with smooth muscle cells and nerve fibres and they, like GIST, express KIT. Differentiation only in myogenic direction without expression of KIT is an indication of smooth muscle origin (e.g. LM or LMS) and solitary differentiation in a neurogenic direction can be suggestive of a tumour of neural origin (e.g. peripheral nerve sheath tumour)(1). Tumours otherwise resembling GIST, but showing no KIT expression, were reported in veterinary (7) and human patients (10,11). These tumours were classified as GIST-like tumours in this report (9) to distinguish them from ‘true’ GIST. Staining intensity, for the IH analysis, were observed to range from very intense with strong punctuated or fibrillar staining to a vaguely positive cytoplasmic background staining, sometimes even within the same tumour. The IH results in this study show similarities with previous reports in dogs (2,7) but also major differences. This may be explained by differences in antibody manufacturers and production methods, antibody (in)compatibility with canine tissue, suboptimal antigen preservation, suboptimal or abnormal antigen expression in tumours and population differences (7). In this study, no significant influence on prognosis after surgical removal could be identified based on tumour type (LM, LMS, GIST or GIST-like), histological or IH characteristics. Even dogs with tumours with very malignant histological appearance can expect long-term survival if complete excision of the tumour is accomplished. Since results of trials with imatinib mesylate seem promising in humans (12), determining KIT expression may prove to be of clinical importance in case of unresectable masses, recurrences, and/or metastases in dogs for future treatment. References: 1. Head KW, Cullen JM, Dubielzig RR, et al. Histologic Classifications of Tumors of the Intestines in Domestic Animals. In: Head KW, Cullen JM, Dubielzig RR, et al eds. Histological Classifications of Tumors of the Alimentary System of Domestic Animals (ed 2), Vol 10. Washington DC: Armed Forces Institute of Pathology, 2003; 87-110. 2. LaRock RG, Ginn PE. Immunohistochemical staining characteristics of canine gastrointestinal stromal tumors. Vet Pathol 1997;34:303-11. 3. Jones TC, Hunt RD, King NW. Microscopic features of benign and malignant neoplasms. In: Cann C, Hunsberger S (eds). Veterinary Pathology (ed 6). Maryland: Williams & Wilkins, 1997; 916. 4. Miettinen M, Fetsch JF. Evaluation of biological potential of smooth muscle tumours. Histopathology 2006;48:97-105. 5. Cooper BJ, Valentine BA. Tumors of smooth muscle. In: Meuten DJ (ed): Tumors in domestic animals (ed 4). Iowa: Iowa State Press, 2002; 319-33. 6. Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol 2002;33:459-65. 7. Frost D, Lasota J, Miettinen M. Gastrointestinal stromal tumors and leiomyomas in the dog: a histopathologic, immunohistochemical, and molecular genetic study of 50 cases. Vet Pathol 2003;40:42-54. 8. Koh JS, Trent J, Chen L, et al: Gastrointestinal stromal tumors: overview of pathologic features, molecular biology, and therapy with imatinib mesylate. Histol Histopathol 2004;19:565-74. 9. Maas CPHJ, ter Haar G, van der Gaag I, Kirpensteijn J. Reclassification of small intestinal and cecal smooth muscle tumors in 72 dogs: Clinical, histologic, and immunohistochemical evaluation. Vet Surg 2007;36:302-13. 10. Debiec-Rychter M, Wasag B, Stul M, et al. Gastrointestinal stromal tumours (GISTs) negative for KIT (CD117 antigen) immunoreactivity. J Pathol 2004;202:430-8. 11. Medeiros F, Corless CL, Duensing A, et al. KIT-negative gastrointestinal stromal tumors: proof of concept and therapeutic implications. Am J Surg Pathol 2004;28:889-94. 12. Gold JS, Dematteo RP. Combined surgical and molecular therapy: the gastrointestinal stromal tumor model. Ann Surg 2006;244:176-84.