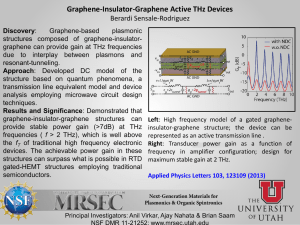
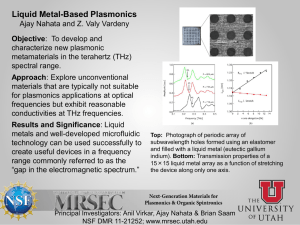
Probing the coupled protein – solvent dynamics by
advertisement

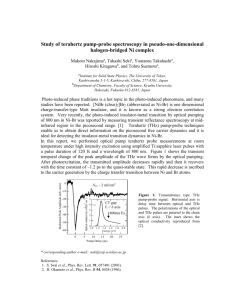
THz sources and THz applications – an overview M. Havenith Physical Chemistry II, Ruhr University Bochum, NC 7/74, 44780 Bochum, Germany martina.havenith@rub.de In recent years the THz range has been closed. Within the last decades new sources have been developed in the opitcal community. These involved photonic sources as well as electronic table top sources. More recently THz FEL has been made available to researches. In the first part of the lecture I will talk about the main principles over the variety of THz systems in this previously difficult frequency range over available THz sources. I will also give an overview about distinct applications ranging from THz imaging to the detection of counterfeit drugs . Observing the rattling modes of ions in the THz Range M. Havenith Ruhr-Universität Bochum The details of ion hydration still raise fundamental questions relevant to a large variety of problems in chemistry and biology. The concept of water "structure breaking" and "structure making" by ions in aqueous solutions has been invoked to explain the Hofmeister series introduced over 100 years ago, which still provides the basis for the interpretation of experimental observations, in particular the stabilization/destabilization of biomolecules. Recent studies, using state-of-the-art experiments and molecular dynamics simulations, either challenge or support some key points of the structure maker/breaker concept, e.g. regarding long ranged ordering/disordering effects. Here, we focus on how systematic terahertz absorption spectroscopy and molecular dynamics simulation study of a series of aqueous solutions of salts, adds a new piece to the puzzle. Concentration dependent THz absorption studies of solvates ions allow to determine the dynamical hydration shell of anions as well as cations. Exmples of monovalent, divalent as well as trivalent salts will be presented. When dissecting the spectrum of trivalent salts we see the influence on cation due to counter anions ranges from self-confinement of both ions to solvent shared ion-pairing, with increasing salt concentration. Reference: S. Funkner, G. Niehues, D.A. Schmidt, M. Heyden, G. Schwaab, K.M. Callahan, D.J. Tobias, M. Havenith Watching the low frequency motions in aqueous salt solutions – the terahertz vibrational signatures of hydrated ions JACS 134, 1030-1035 (2012). Some like it cool – THz absorption studies of as a tool to study biomolecular hydration Martina Havenith Ruhr-Universität Bochum The details of hydration still raise fundamental questions relevant to a large variety of problems in chemistry and biology. We have shown the THz spectroscopy in combination with MD simulations is a powerful took to study the sub-nsec hydration. THz spectroscopy is also able to reveal the important role of hydration on biomolecular function: Antifreeze proteins (AFPs) are specific proteins which are able to lower the freezing point of aqueous solutions relative to the melting point. They are preferential docking to ice nano ice crystals thereby preventing further growth of these. Whereas the antifreeze acitivity for several of these AFP has been characterized so far, the molecular mechanism is still a matter of controversial discussion: By a combination of THz absorption spectroscopy and MD simulations we could show that the activity of AFPs can be attributed to two distinct molecular mechanisms: a) short range direct interaction of the protein surface with the growing ice face and b) long range interaction via protein-induced water dynamics extending up to 20 Å from the protein surface. We propose a long range retardation of the H-bond dynamics with a gradient towards the ice binding site. A similar gradient in the H-bond dynamics was found by us for enzymes near the catalytic site. We will discuss the underlying molecular mechanism supporting the docking at specific sites. References: M. Heyden, E. Bründermann, U. Heugen, G. Niehues, D.M. Leitner, M. Havenith, Long range influence of carbohydrates on the solvation dynamics of water – Answers from THz absorption measurements and molecular modeling simulations, J. Am. Chem. Soc. 130, 5773-5779 (2008) M. Grossmann, B. Born, M. Heyden, D. Tworowski, G.B. Fields, I. Sagi, M. Havenith, Correlated structural kinetics and retarded solvent dynamics at the metalloprotease active site, Nature Structural & Molecular Biology 18, 1102-1108 (2011). S. Funkner, G. Niehues, D.A. Schmidt, M. Heyden, G. Schwaab, K.M. Callahan, D.J. Tobias, M. Havenith, Watching the low frequency motions in aqueous salt solutions – the terahertz vibrational signatures of hydrated ions, JACS, 134, 1030-1035 (2012). K. Meister, S. Ebbinghaus, Y. Xu, J.G. Duman, A. DeVries, M. Gruebele, D.M. Leitner, M. Havenith, Long-range protein-water dynamics in hyperactive insect antifreeze proteins, Proc. Natl. Acad. Sci. USA 110(5) 1617-1622 (2013). Watching the dance of water in the hydration shell of ions and biomolecules in the THz frequency range M. Havenith Physical Chemistry II, Ruhr University Bochum, NC 7/74, 44780 Bochum, Germany martina.havenith@rub.de In recent years a new frequency window has been opened: The THz range. In pioneering studies it could be shown that THz absorption spectroscopy is a new tool to study the solvation dyncamics of biomolecules [1]. THz spectroscopy probes sensitively the fast (sub-psec) collective network dynamics of bulk water. Accompanying ab initio MD simulation unravel the underlying molecular motions: In contrast to the mid infrared regime -where the absorption peaks can be assigned to intramolecular motions- in the frequency regime below 1000 cm-1 intermolecular motions with concerted particle motions dictate the spectrum [2]. Precise measurements of absorption coefficients of solvated solutes in the THz regime allow now a precise view on changes in hydration dynamics of solutes during biological function [3,4,5]. [1] S. Ebbinghaus, S.J. Kim, M. Heyden, X. Yu, U. Heugen, M. Gruebele, D.M. Leitner, M. Havenith, "An extended dynamical solvation shell around proteins," Proc. Natl. Acad. Sci. USA, 104, 20749 (2007). [2] M. Heyden, J. Sun, S. Funkner, G. Mathias, H. Forbert, M. Havenith, D. Marx Dissecting the THz spectrum of liquid water from first principles via correlations in time and space. Proc. Natl. Acad. Sci. USA, 107, 12068 (2010). [3] D.A. Schmidt, Ö. Birer, S. Funkner, B. Born, R. Gnanasekaran, G. Schwaab, D.M. Leitner, M. Havenith, Rattling in the cage: Ions as probes of sub-ps water network dynamics, J. Am. Chem. Soc., 131(51), 18512-18517 (2009) [4] S.J. Kim, B. Born, M. Havenith, M. Gruebele, Real-time detection of protein-water dynamics upon protein folding by terahertz absorption, Angewandte Chemie Intl. Edition 47 (34), 6486-6489 (2008). [5] M. Grossmann, B. Niehues, M. Heyden, D. Tworowski, G.B. Fields, I. Sagi, M. Havenith (2010), Correlated structural kinetics and retarded solvent dynamics at the metalloprotease active site, Nature Structural & Molecular Biology, published online 18.9.2011; doi:1038/nsbm.2120