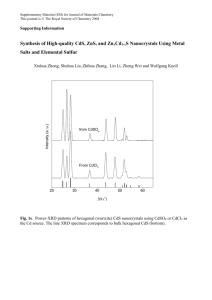
cadmium vapor removal from incineration flue gas by various
advertisement

CADMIUM VAPOR REMOVAL FROM SILMULATED INCINERATION FLUE GASES BY VARIOUS ADSORBENTS LU Huan-liang , WANG Wei (Dept. of Environmental Science & Engineering,Tsinghua University,Beijing 100084, China) E-mail: luhuanliang@tsinghua.org.cn Abstract :The volatile heavy metal Cd released from municipal solid waste incinerator threatens human health. The injection of suspended mineral substrates can effectively adsorb heavy metal vapor. Five different materials were utilized: light calcium carbonate, diatomite, kaolin, attapulgite clay and α-Al2O3. Light calcium carbonate was modified with two methods:1) thermally treated to obtain lime products with more BET specific areas;2)thermally treated by adding NaCl solution as modifier . A static adsorption system with a simulation flue gas cleaning system was developed to quantify their removal capabilities with CdCl2 vapor. The results show that each adsorbent can remove a certain amount of Cd vapor, and the modified substrates are more effective than the five absorbents and CaO/NaCl exhibits almost the most effectiveness. In addition, the atmosphere of the system influences the adsorption capacity, and the mass of CdCl2 captured in the simulated flue gases is larger than that in nitrogen. Keywords: simulated flue gaess; non-metallic minerals; CdCl2 vapor; adsorption 1. Introduction The emission of heavy metals to the environment is much concerned due to their associated health hazard. During municipal solid waste incineration, a considerable part of the volatile heavy metals like Hg, Cd, Pb is primarily entrained onto fine ash particles[1,2]. Current air pollution control equipments such as fabric filter and electrostatic precipitators can not effectively capture these pollutants. As a result, the volatile fraction of these metals can pass the particle filter and release into the environment. An additional unit is therefore urgently needed to remove the gaseous heavy metals from the flue gas. Adsorption is regarded as one of the most efficient ways. Uberoi and Shadman [3] investigated the adsorption of cadmium onto different types of adsorbent at 800℃in a simulated flue gas atmosphere of CO2, O2, N2 and H2O. The results showed that the degree of adsorption depended on the type of substrate. The interaction of cadmium with kaolin resulted in the formation of a cadmium aluminum silicate is also indicated, so the overall sorption process was not just physical adsorption but a complex combination of adsorption and chemical reaction. Wey et al [4]examined the adsorption characteristics of organic compounds and heavy metals at various incineration conditions. The results indicated that activated carbon adsorbed more efficiently than zeolite, and temperature only slightly influenced the removal efficiency of metals. However, activated carbon costs much higher than other mineral adsorbents despite of its high removal efficiency so other cheaper adsorbents will be preferred and developed. In this paper, a simulated flue gas reactor with a quartz tube is developed. Some mineral adsorbents are also modified to capture Cd pollutant. The results show that the adsorption of heavy metal species relates to flue gas conditions and the modified calcium carbonate exhibits almost the same effectiveness as activated carbon. Hence, lime should be considered as a possible alternative to relatively expensive activated carbon. 2. Experimental 2.1 Materials The materials, or rather some mineral adsorbents are listed in the Table1, and their BET specific areas, total pore volumes and average pore diameters were measured. To obtain more specific area absorbents, the light calcium carbonate was modified with two methods:1) thermally treated to obtain lime products with more BET specific areas;2)thermally treated by adding NaCl solution as modifier and lime was thus obtained in the laboratory at high temperatures during different times as shown in Table2. All samples were sieved into 125-250 mesh. To avoid further recarbonation and water absorption, the samples were kept in a desiccator and manipulated using a glove bag. TABLE 1. Composition and characterization of the absorbents Absorbents Calcinated kaolin Attapulgite clay Diatomite α-Al2O3 Calcium carbonate Composition/% SiO2 53.72 Al2O3 44.98 SiO2 52-61 Al2O3 10-12 SiO2 89.01 Al2O3 95 CaCO3 98 BET specific area /m2 ·g-1 9.690 131.4 4.545 5.219 11.67 Total pore volume /ml· g-1 0.0304 0.313 0.00671 0.00664 0.0194 Average pore diameter /nm 12.56 9.525 5.908 5.091 6.643 TABLE 2. Treating conditions and characterization of lime samples specific area (m2/g) sample size (μm) Treatment conditions Lime(1) 60-125 850℃ 24h in air 16.23 Lime(2) 60-125 900℃ 6h in air 18.27 Lime(3) 60-125 950℃ 12h in air 13.82 Lime(4) 60-125 5%NaCl 900℃ 6h 5.30 Lime(5) 60-125 10%NaCl 900℃ 6h 3.96 Lime(6) 60-125 25%NaCl 900℃ 6h 3.92 2.2 Apparatus and experimental procedure Fig. 1 shows a schematic diagram of a quartz tube heated by electrical furnace. Three small crucibles were placed in the tube and contained a melt of CdCl2. The temperature inside the quartz tube was varied from the melting temperature of CdCl2 to a point constantly remaining far below the boiling point at atmospheric pressure (1000K). In order to avoid the influence of other components in the flue gas, the inlet gases to stimulap incineration conditions included air and nitrogen. The 1.00 m3/h simulated flue gases (see Table 3)was controlled by their respective flow meter. The particles of the mineral substrates were placed in the bottom of the quartz tube. The CdCl2 vapor was then filtered and obsorbed by the particles. In the end, the outlet gases were passed through a sampling train consisting of a series of nitric acid and water bubblers to trap the unabsorbed CdCl2 species. 3 I-7 I-8 I-5 I-6 6 2 V-4 V-5 V-3 V-2 5 O2 4 1 SO2+N2 CO2+N2 HCl+ N2 O2+N2 CdCl2 Figure 1 Static adsorption diagram of CdCl2 vapor 1-simulated flue gases; 2-pressure-relief valve; 3-mass flow meter; 4-thermocouple; 5-quartz boat; 6-cartridge; 7-adsorption solution 7 TABLE 3. Components of the simulated MSW flue gases Components Concentration(V/V) O2 6% CO2 12% SO2 60~100ppm HCl 300~500ppm Cd 50mg/m3 N2 Balance gas 2.3 Methods of adsorption, sampling, and analysis The CdCl2 compound in the absorbent was extracted by soxhlet method using HNO3 (1+1) and H2O2 (30%) as solvents and fluxed the solution more than 3 hours. The dissolved solution was then analyzed by atomic absorption spectrometry (AAS) method. 3 Results and Discussion 3.1 Evaluation of CdCl2 vapor pressure As a limiting condition, the vapor pressure at the CdCl2 surface in the crucible was calculable from classical thermodynamic relationships[5]: for solid CdCl2 (T ≤841.65 K) lgP =-10179 T-1-2.79 lgT+17.689 for liquidCdCl2 (T≥841.65 K) lgP= -9335 T-1 -5.83 lgT+ 25.579 Fig.2 shows the relationship between vapor pressure of CdCl2 versus furnace temperature in the range of 500900K, showing that the critical point of vapor emission is 900K and the vapor pressure dramatically increase with the temperatures. The data from the experiment shows a good agreement to that from the theoretical calculations. Figure 2 Vapor pressure of CdCl2 using thermodynamic analysis (┅ ★┅ Theoretical data from thermodynamic analysis ◆Experimental data) 3.2 Effect of temperature on the CdCl2 sorption on the tested adsorbents. Fig. 3 lists the mass of CdCl2 captured by calcium carbonate at a pressure of 100Kpa. With the increase of the temperature, the adsorption mass of Calcium carbonate and diatomite goes up steadily and reaches its peak at 1000K. The experiments after that will use 1000K as the treating temperature. 3.3 Effect of time on the CdCl2 sorption on the tested adsorbents. Fig. 4 lists the mass of CdCl2 captured by calcium carbonate at a pressure of 100Kpa. The contacting time has influenced the adsorption mass of Calcium carbonate and diatomite before 20 min, before reaching to its peak. The following experiments will use 30min as the treating time. Figure 3 Effect of temperature on adsorption capacities of CdCl 2 vapor Figure 4 Effect of time on adsorption capacities of CdCl 2 vapor 1-CaCO3; 2- Diatomite Adsorption capacity(mg/g) 3.4 Adsoption mass of CdCl2 on the tested adsorbents The removal capacities of CdCl2 in gaseous phase defined as (concentration of pollutants before adsorbed— concentration of pollutants after adsorbed by adsorbents )/weight of adsorbent, and the results of the tested adsorbents have been shown in Fig. 5. 600 Flue gas 500 N2 400 300 200 100 0 A B C D E Figure 5 Mass of CdCl2 vapor captured by various absorbents at different atmospheres A:Calcium carbonate; B: Diatomite; C:Calcinated kaolin;D: Attapulgite clay;E:α-Al2O3 It can be seen from Fig.5 that the five adsorbents can removal a certain amount of Cd vapor and light calcium carbonate is the more effective compared with any other absorbent. Furthermore, the atmosphere of the system influences the adsorption capacity, and the mass of CdCl2 captured in the air is larger than that in nitrogen. The results in Fig.6 indicate that the removal capacity of Cd vapor by lime is higher than unthermally-treated calcium carbonate. Lime (2), which was adsorption capacity(mgCd•g -1 sorbent) thermally treated at 900℃ in air for 6 h is more effective compared with limes in other treating conditions due to its largest BET specific area(see TABLE2).However, as the temperature above 950℃ will sinter the mineral and disrupt the inner pore space of calcium carbonate.Fig.6 also indicated that the adsorption performance of lime substrates obtained from adding NaCl (Lime 4 and Lime 5)is the best of all, which may due to the CaO/NaCl layer generated during the modification. 800 700 600 500 400 300 200 100 0 0# 1# 2# 3# 4# 5# 6# 7# Figure 6 Mass of CdCl2 vapor captured by lime series 0#:Calcium carbonate; 1#-6#: lime(1)-lime(6);7#:CaO (A.R.) 4 Conclusions Five different types of mineral adsorbents, three types of thermally treated calcium carbonate(lime products), have been used in this study to investigate the removal efficiencies of Cd vapor from incineration flue gas. Each adsorbent can remove a certain amount of Cd vapor.The optimal adsorption conditions in this study should be temperature-1000K,and time-25min. The thermally treated products lime exhibits higher adsorption ability than calcium carbonate and other minerals. Individual removal capacities of lime for Cd vapor are found to be above 120mg/g. In addition, the atmosphere of the system influences the adsorption capacity, and the mass of CdCl 2 captured in the air is larger than that in nitrogen. References [1] Chen J.C., Wey M.Y., Liu Z.S. ADSORPTION MECHANISM OF HEAVY METALS ON SORBENTS DURING INCINERATION[J], J. ENVIR. ENGRG. ,ASCE,2001, 127 (1):63-69. [2] EVANS J. , WILLIAMS, P. T. HEAVY METAL ADSORPTION ONTO FLYASH IN WASTE INCINERATION FLUE GASES[J]. Trans IChemE, Part B,2000,78(1):40-46. [3] Uberolt M., Shadman F. High-Temperature Removal of Cadmium Compounds Using Solid Sorbents[J]. Environ. Sci. Technol.,1991, 25(7):1285-1289. [4] Wey M.Y., Jyh L. Y., Jou S.I., Chiang B.C., Wei M.C. ADSORPTION ON CARBON AND ZEOLITE OF POLLUTANTS FROM FLUEGAS DURING INCINERATION[J], J. ENVIR. ENGRG., ASCE,1999, 125(10): 925-927 [5] R. Masseron, R. Gadiou, L . Delfosse. Study of the Adsorption of CdCl2 Vapor on Various Minerals Using a Drop Tube Furnace[J]. Environmental Science and Technology,1999,33(20):3634-3640.