Equilibrium Practice Problems (Ch
advertisement

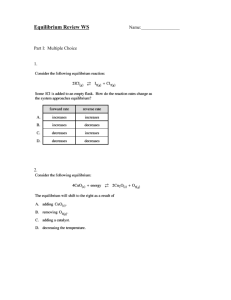
Equilibrium Practice Problems – Set 1 1) Write the expression for the equilibrium constant, Keq, for the following reactions: a) 2H2S(g) 2H2 (g) + S2 (g) b) 2N2O5(g) 4NO2(g) + O2 c) 4NO(g) + 2O2 d) 2NO(g) + Br2 e) CO(g) + 2H2 f) SO2 (g) (g) + NO2 2N2O4(g) 2NOBr(g) (g) (g) (g) CH3OH(g) (g) SO3(g) + NO(g) 2) Calculate the equilibrium expression for equation 1a if the equilibrium concentrations are [H2S] = 0.25 mol/L, [H2] = 0.88 mol/L, [S2] = 0.44 mol/L. 3) Calculate the equilibrium expression for equation 1b if the equilibrium concentrations are [N2O5] = 0.50 mol/L, [NO2] = 0.80 mol/L, [O2] = 0.20 mol/L. 4) I2 (g) + Cl2 (g) <===> 2ICl(g) Equal molar quantities of iodine and chlorine combine to form iodine choloride. The equilibrium constant for this reaction is 9.8 at a certain temperature, and the equilibrium mixture contains 1.44 mol ICl. How many moles of I 2 are present in the equilibrium mixture? 5) The equilibrium constant for this reaction is 5.6: 2NO2 (g) <===> N2O4(g) In a 1.00 L container the amount of N2O4, at equilibrium, is 0.66 mol. What is the equilibrium concentration of NO 2? 6) Which value of Keq indicates most favorablity of product formation: K eq = 1 x 1012; Keq = 1.5; Keq = 5.6 x 10-4. 7) Hydrogen sulfide gas decomposes into its elements and establishes an equilibrium at 1400°C. 2H2S(g) <===> 2H2(g) + S2 (g) A 1.00 L sample of this gas mixture contains 0.18 mol H 2S, 0.014 mol H2, and 0.035 mol S2. Calculate the equilibrium constant, Keq, for this reaction. 8) A 1.00 L flask contains 4.40 mol of Hl at a certain temperature. The K eq at this temperature is 5.0 x 10 - 4. What are the concentrations of H2 and I2 at equilibrium? 2Hl(g) <===> H2(g) + I2(g) (hint: equal molar concentrations of H2 and I2) 9) Suppose that 2.00 mol of HNO2 is dissolveed in 1.00 L of water at 25°C. Because HNO 2 is a weak acid, it is in equilibrium with ions, H+ and NO2-. What are the concetrations of these two ions at equilibrium? HNO2(aq) <===> H+(aq) + NO2-(aq) Keq = 4.47 x 10-4 10) We place some nitrogen and hydrogen in an empty 5.00 liter vessel at 500°C. When equilibrium is established, 3.01 mol N2, 2.10 mol H2, and 0.565 mol of NH3 are present. Calculate Keq for the following reaction at 500°C: N2 (g) + 3H2 (g) 2NH3(g) 11) At a very high temperature, Keq = 1.0 x 10-13 for the following reaction: 2HF(g) H2 (g) + F2 (g) At a certain time the following concentrations were detected: [HF] = 0.500 M; [H2] = 1.00 x 10-3 M; [F2] = 4.00 x 10-3 M a) Is the system at equilibrium? b) If not, what must occur for equilibrium to be established? 12) The reaction between nitrogen and oxygen to form NO(g) is represented by the following chemical equation: N2 (g) + O2 (g) 2NO(g) The equilibrium concentrations for the gases at 1500 K are: [N2] = 6.4 x 10-3 M; [O2] = 1.7 x 10-3 M; [NO] = 1.1 x 10-5 M Calculate the value of Keq at 1500 K from these data. 13) At elevated temperatures, BrF5 establishes the following equilibrium: 2BrF5(g) Br2 (g) + 5F2 (g) The equilibrium concentrations of the gases at 1500 K are: [BrF5] = 0.0064 M; [Br2] = 0.0018 M; [F2] = 0.0090 M. Calculate Keq. 14) For the reaction: Cl2 (g) + F2 (g) 2ClF(g) , Keq = 19.9. What will happen in a reaction mixture originally containing [Cl2] = 0.2 M, [F2] = 0.1 M, and [ClF] = 3.65 M?