Bloodborne Pathogens Hepatitis Vaccination Guidelines
advertisement

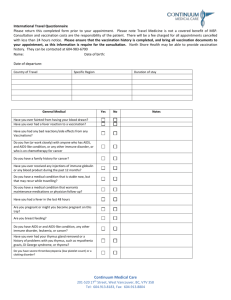
USC Bloodborne Pathogens Hepatitis B Vaccination Guidelines I. Policy This guideline will comply with the Occupational Safety and Health Administration (OSHA) Bloodborne Pathogen Standard (BBP) 29 CFR 1910.1030. Universal precautions will be utilized to prevent contact with blood or other potentially infectious materials. Under circumstances in which differentiation between body fluid types is difficult or impossible, all body fluids shall be considered potentially infectious materials. II. Scope and Application USC’s exposure control plan applies to all occupational exposure to blood or other potentially infectious materials at all USC campuses and related facilities and operations. Each campus, operation, or department will be responsible for training, documentation, implementation, and recordkeeping for their BBP exposure control plan. III. IV. Hepatitis B Vaccination (HBV) Procedure for Non-Research Personnel 1. Employees who are offered the Hepatitis B vaccination, must complete the Clinical Immunization Requirements Information Form EHS-F-146. The employee must fill out the form, obtain their supervisor’s approval, check the appropriate box for previously vaccinated, have not been vaccinated but wish to receive, or wish to decline. The employee must sign the form, and send the form to USC Environmental Health and Safety for final approval. 2. Auxiliary departments, such as USC-Columbia Housing will be required to provide a cost code to Thomson Student Health Center for personnel from their department to pay for Hepatitis B vaccinations. Employees in Job Classification I who may be routinely exposed to bloodborne pathogens or other potentially infectious materials will be offered the Hepatitis B vaccinations. These departments should also evaluate and offer these vaccinations to employees in Job Classification II who may be exposed to bloodborne pathogens or other potentially infectious materials under certain conditions. Hepatitis B Vaccination (HBV) Procedure for Research Personnel 1. The Institutional Biosafety Committee (IBC) has established a requirement that all faculty, staff or students that work with any of the human materials indicated below or have a potential occupational exposure to hepatitis B virus (HBV) when conducting University research must complete the HBV vaccination series or provide proof of HBV vaccination before starting work: Human blood, human blood components, and products made from human blood Semen, vaginal secretions, cerebrospinal fluid, synovial fluid, pleural fluid, pericardial fluid, peritoneal fluid, amniotic fluid, saliva in dental procedures, any body fluid that is visibly contaminated with blood, and all body fluids in situations where it is difficult or impossible to differentiate between body fluids; Any unfixed tissue or organ (other than intact skin) from a human (living or dead) HIV-containing cell or tissue cultures, organ cultures, and HIV- or HBV-containing culture medium or other solutions; and blood, organs, or other tissues from experimental animals infected with HIV or HBV 2. Any necessary HBV screening will be provided by the Thomson Student Health Center Immunization Clinic. If an individual with potential for exposure decides to decline the vaccination due to personal or medical reasons (e.g. contraindication) they will not be approved to conduct research involving occupational exposure to HBV. 3. Researchers must download and complete the Hepatitis B Vaccination Policy for Research Laboratories which is available on the USC EHS webpage under Bloodborne Pathogens.