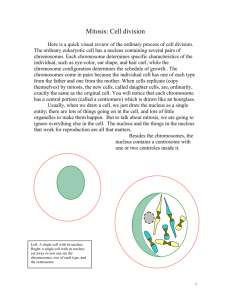
Gehin Charlotte Master 1 ENS LYON MODIFICATION OF THE SPD
advertisement

Gehin Charlotte Master 1 ENS LYON QuickTi me™ et un décom presseur T IFF (non compressé) sont requis pour visi onner cett e i mage. MODIFICATION OF THE SPD-2 PROTEIN AND ITS ROLE DURING CENTROSOME ELIMINATION IN C.elegans The Roy laboratory – Developmental control of the cell cycle Mc GILL UNIVERSITY - MONTREAL Version finale Version finale 26 400 caratères Gehin Charlotte Master 1 ENS LYON MODIFICATION OF THE SPD-2 PROTEIN AND ITS ROLE DURING CENTROSOME ELIMINATION IN C.elegans Sexually reproducing organisms must eliminate a pair of centrosomes prior to the first zygotic division to avoid the formation of a multipolar spindle. During the development of the C. elegans germ line, germ cells undergo several developmental changes following oocyte specification that include the elimination of the centrioles that were present during the earlier meiotic stages. This process requires the activity of a p21/p27-like CDK inhibitor protein called cki-2. Moreover, a cyclin E/CDK2-dependent phosphorylation is required to stabilize the centrioles and its cki-2-dependent inhibition results in their rapid destabilization/elimination. Many of the proteins required for centrosome duplication possess canonical CDK phosphorylation sites and could be candidate proteins for a potential role as CDK targets for centriole stabilization. Among them, SPD-2, acting upstream the centriole assembly pathway is able to shift from perinuclear foci to nuclei during oogenesis, suggesting that it is modified in such a way that influences its localization. INTRODUCTION Centrosome and centrioles Sans titre1In animal cells, the function of microtubule-organizing centers (MTOCs) is mainly assumed by centrosome (1). This organelle, discovered at the turn of the twentieth century and first studied by Theodor Boveri (2) comprises, as illustrated in Figure 1, two distinct structures: a pair of centrioles, the main core constituents of the centrosome, surrounded by a filamentous network of proteins, called pericentriolar material (PCM). Centrioles play a crucial role in PCM assembly and consequently, in determining the MTOC number (1). Molecular epistasis experiments, which have Figure 1. A: C. elegans centrosome structure. B: Sequential recruitment of proteins during C. elegans centriole assembly: SPD-2, which localizes both to PCM (PeriCentriolar Material) and centrioles, recruits ZYG-1 kinase to the centriole Together, they make it possible the incorporation of structural components of the central tube: SAS-5 and SAS-6. Finally, SAS-4 supports the tethering of microtubules to the central tube, allowing PCM recruitment and organization (3). Gehin Charlotte Master 1 ENS LYON been successfully performed in C. elegans for a few years, showed that their assembly required the sequential action of four coiled-coil proteins and one kinase: SAS-4, -5, -6, SPD-2 (SPindle Defect) and ZYG-1 (ZYGote defective embryonic lethal), respectively (Figure 1). Even though centrioles are different in nematodes compared to Vertebrates, because of their short size, their singlets of microtubules (instead of doublets) and their lack of –Tubulin and Centrin (two major centrosome proteins in Vertebrates), C. elegans has emerged in recent years as an attractive model system for studying centrosome duplication. Using genetic analyses of events that occur in the one-cell embryo in C. elegans, researchers were able to identify essential components for centriole formation; in some of which, including SAS-4, SAS-6 and SPD-2, share homologies with centriolar proteins of mammals. The morphological properties of the one-cell stage embryo and a clear size difference between centrioles and the PCM allow analysis of centrosome duplication in living specimens with high resolution as well as studies of gene function of centrosome components (by fluorescence imaging or by RNA interference), respectively (4). Centrioles duplication and cell cycle Centrioles from both nematodes and other metazoans play crucial roles in cellular mechanisms. Their main function is to provide a strong solid core around which more amorphous PCM can be structured, a template structure needed to produce a new centriole, or an anchor for microtubules in the PCM, which communicate force from the cortex to the nucleus and then along the spindle guaranteeing that equivalent forces will be exerted from all sides onto the attachment point of these microtubules during cytokinesis. Moreover, centrioles act as a cell cycle regulator consistent with their role as a Figure 2. Centriole and centrosome duplication during cell cycle. In red: checkpoints of cell cycle ; in green, blue and pink: other controls of centrosome duplication; in black: phases of the cell cycle scaffold onto which signaling molecules may be concentrated and coordinated (5). Such functions suggest that their number Gehin Charlotte Master 1 ENS LYON must be strictly regulated and intimately linked to the cell cycle (6). While the mechanism of their duplication is still only partially understood (4), it was shown that different levels of controls, involving some kinases, occur throughout the cell cycle. Firstly, the centriole-centrosome duplication cycle seems to be regulated by canonical checkpoints of the cell cycle, as illustrated by the red stars on Figure 2. Secondly, two rules could be determined: on the one hand, centrosomes duplicate once and only once per cell cycle, in synchrony or not with DNA during the S phase (1,6). This property is ensured by Separase (Figure 2, in green), a protease that prevents a new round of duplication from S phase to mitosis. On the other hand, in order to avoid the production of excessive numbers of centrioles, a phenotype commonly observed in cancer cells, there is only one progeny centriole next to each parental centriole, thanks to the combined actions of the ZYG-1 kinase and the Cdk-2/cyclin A and/or E complex (Figure 2, in pink and blue). Finally, some phosphatases are expected to counteract these kinase actions. (6) In addition, centrosome biogenesis can follow a route different from the canonical centrosome duplication cycle, as described in Figure 2. This is the case in cancerous cells in Mammals and during normal development in other metazoans: such as endocycling cells in Drosophila or the intestinal cells and spermatocytes in C. elegans. In animals, fertilization requires the fusion of two haploid gametes resulting in a zygote containing only one full centrosome, so that its first mitosis leads to the formation of two centrosomes. This result can be obtained by different strategies depending on the species. In C. elegans, the centrioles disappear during oogenesis and spermatocytes lose their PCM (4). As in other species, the mechanism for centriole disappearance is unknown. However, it was recently shown by genetic experiments that a p21/p27-like cyclindependent kinase (Cdk) inhibitor (Cki-2) was needed for maternal centriole elimination during oogenesis. Indeed, loss of cki-2 results on the one hand in misregulation of cyclin E/Cdk-2 activity, and on the other hand in the inheritance of centrosome during oogenesis, suggesting that targets of Cdk activity are involved in centriolar perdurance (7). Spd-2 It was previously shown that in C. elegans deficient for cki-2 by cosuppression reverse genetic experiments (cki-2cs), SPD-2 persisted throughout oogenesis (7). Bioinformatic analysis of proteins needed for centriole assembly demonstrated that all the proteins (SAS-4, SAS-5, SAS-6, ZYG-1 and SPD-2) involved in centriole assembly contained a pattern of Cdk-phosphorylation sites (annexe, Figure 8). Therefore, might the SPD-2 coiled—coil protein (known to act upstream of the centriole assembly pathway) be a substrate for cyclin E/Cdk-2 and by this way act upstream of centriolar disassembly? Recently, a study describing SPD-2 functional domains has briefly shown that Gehin Charlotte Master 1 ENS LYON varying amounts of SPD-2 could be present in the nuclei of oocytes (8, Figure 3A) whereas centrioles have already disappeared from the cytoplasm and whereas SPD-2 clusters generally appear as perinuclear foci in dividing cells (8). We can thus wonder if SPD-2 is modified during the cell cycle and what its role is if it is involved in centrosome elimination. To answer these questions, my first work was to confirm a change in SPD-2 localization from the cytoplasm to the nucleus by immunofluorescence, and, if confirmed, at what step of oogenesis the shift occurs. Then, I attempted to discover what modification allowed this move. Using Western Blots, I tried to identify different forms of denatured SPD-2 protein but I have not managed to demonstrate it yet because of some experimental constraints. Then, I envisaged to express a genomic spd-2 sequence comprising a mutated phosphorylation site using bombardment, to start a new immunofluorescence assay and to perform a new Western Blot using SPD-2 antibodies in order to determine if the shift observed was really due to a phosphorylation event. However, this experiment is still underway. RESULTS Spd-2 shifts from the cytoplasm to the nucleus during oogenesis My objective was to look for the presence of SPD-2 coiled-coil protein in the nucleus of oocytes (8). I performed an immunofluorescence labelling of SPD-2::GFP in dissected and fixed gonads from adult spd-2::spd-2::GFP worms using monoclonal antibodies against GFP (Figure 4). By microscopy, I could identify SPD-2::GFP in the germline but also, later, in the pachytene, where centrosome are absent (Figure 3). Moreover, It was shown that SPD-2::GFP foci shifted from a perinuclear region in the hermaphrodite mitotic germ cells (Figure 4, A) to the nuclei of late pachytene cells during oogenesis (Figure 4, B). Figure 3. Adult hermaphrodite germline of C. elegans. Extruded gonads stained with DAPI. At adult stage, wild-type hermaphrodite C.elegans worms produce both female and male gametes. The female germ line is divided into: a "distal mitotic" region made of dividing germ cells, a "transition zone" where germ cells enter meiosis and form a germline syncitium, a "pachytene zone" where the meiotic syncitium grows and where DNA begins condensing. Finally, in proximal zone, cells undergo diakinesis to become oocytes, i.e cells arrested in meiotic prophase I where six chromosomes are clearly visible (9). Gehin Charlotte Master 1 ENS LYON Figure 4. SPD-2-GFP persists in the later stages of pachytene and shifts from the perinuclei to nuclei during oogenesis. (A-D) extruded gonads from a strain expressing GFP-SPD-2 and stained for GFP (green-sometimes appears yellow due to overlap with red on merged picture). SPD-2 concentrates in perinuclear foci (arrows) in the distal portion of the hermaphrodite germline (A). Such foci are absent in oocytes (B) and shift into nuclei, in late pachytene (B-D). In parallel, an immunofluorescence labeling of SPD-2 in dissected and fixed intestines from different stages of N2 worms was performed with polyclonal antibodies against SPD-2 (Figure 5). By microscopy, it was shown that SPD-2 perinuclear foci, present in diploid (2n) intestinal cells from the L1 stage, shifted into the nuclei in polyploid (8-16n) intestine cells from L2-L3 stage (Figure 5, B), where centrioles are absent (annexe, Figure 9). Gehin Charlotte Master 1 ENS LYON Int 3-7 Figure 5. SPD-2 localization changes from centriolar to nuclear foci at the end of the L2 stage in intestinal nuclei. (A) Post-embryonic cell cycle of intestinal nuclei. The intestinal cells do not undergo proliferative postembryonic cell division. During the L1 molt, some intestine cells (Int 3-7) undergo DNA replication then, perform karyokinesis without cytokinesis. After the karyokinesis, all the nuclei undergo one round of endoreplication. Endoreplication occurs at each of the following larvae molts, so that each of the intestinal nuclei in the adult is 32C (9) . (B) Extruded intestine from N2 larvae stained with polyclonal antibodies against SPD-2. SPD-2 concentrates in perinuclear foci (arrows) in L1 intestine cells. Such foci are absent in L2-L3 while diffuse SPD-2 is visible in intestinal nuclei (unpublished Yu Lu's data). This experiment, done both in the germline and in intestinal cells, confirms the ability of SPD-2 to shift into the nuclei from the perinuclear foci during centriole elimination in C. elegans. SPD-2 coiled-coil protein contains CDK- targeted phosphorylation sites Since SPD-2 acts upstream of centriole assembly and centriole disappearance during oogenesis seems to be linked to the inhibition of Cyclin-dependent kinase 2 by CKI-2 (7), the nuclear form of SPD-2, observed by immunofluorescence, might result from a modification made by Cdk2, meaning that it might be phosphorylated. According to a previous study (11) the presence of six conserved Cdk phosphorylation sites (both in C. elegans and C. briggsae) was expected in the nematode SPD-2 sequence. I confirmed, in silico their presence (aa positions: 143, 171, 220, 233, 259 and 545) in C.elegans SPD-2, using a new Group-based Phosphorylation (GPS) Scoring Gehin Charlotte Master 1 ENS LYON method (12). Moreover, by this way, I could classify the most probable Cdk-phosphorylation sites according to their GPS score (Figure 6). Among the twelve phosphorylation sites found, only one, located on the Threonine 233 had a GPS score higher than 8 (Figure 6). Thus, Thr 233 is the most probable Cdk-phosphorylation site on SPD-2, if considering that conservation of sequences throughout evolution is evidence of function. Figure 6. The most probable Cdk-phosphorylation sites of SPD-2 sequence, found by the Group-based Phosphorylation Site (GPS) scoring method (12). GPS scoring is a statistical method. Best scores correspond to the most probable phosphorylation sites. Threonine 233 is the only amino acid with a GPS score higher than 8. Small stars surmount amino acids most likely to be targeted by CDKs. Only scores higher than 4 were considered as significant. Different forms of SPD-2 protein might coexist during C. elegans development Having previously demonstrated that SPD-2 contains Cdk-phosphorylation sites and that the protein shifts from the cytoplasm to the nuclei in some C. elegans tissues (germ line and intestine), it can be speculated that the passage of SPD-2 into different cellular compartments (cytoplasm and nuclei) during post-embryonic development might be due to a modification of the protein, such as a phosphorylation. In order to determine the presence of any modified SPD-2, I first looked for the presence of different forms of SPD-2 in wild-type worms, by Western Blot, using a polyclonal antibody against SPD-2 (8). In order to know if SPD-2 undergoes modifications specific to the development of the germ line or intestine, I isolated proteins from different larval stages in wild-type C. elegans hermaphrodites. More exactly, I compared SPD-2 from the L1 stage, where there are only mitotic germ cells (dividing cells where centrioles are present, so where SPD-2 is located only in perinuclear foci), to SPD-2 taken from young adults where gonads are completely functional and in which I had observed the presence of SPD-2 from late pachytene nuclei. Moreover, I performed the same experiments with worms in the L3/L4 transition stage, when the oocytes are not yet present, but where pachytene nuclei are well established, unlike the L1 stage. In this way, I could determine wether the quantity of modified SPD-2 is linked to oogenesis (Figure 7). Gehin Charlotte Master 1 ENS LYON spd-2 (RNAi) Figure 7. Western Blot analysis of protein extracts of wild-type C. elegans. Total protein extracts were prepared from L1, L3/L4 and young adult N2 worms, and spd-2(RNAi) animals. 5l (lane 3) or 15l (lane 1 and 2) of protein extract was separated by SDS-PAGE and the presence of SPD-2 and -Tubulin (positive control) was measured by Western blot (primary antibodies: polyclonal anti-SPD-2 (8), anti--Tubulin; secondary antibodies: antirabbit and anti-mouse, respectively) The molecular weight of SPD-2 has been estimated at 91.5kDa (13). However, the bands that observed on the Western Blot indicate 60kDa and 85kDa proteins, regardless of the age considered (Figure 7, lanes 1-3). Moreover, an additional band located at 60-85kDa is visible in young adult worms (Figure 7, lanes 3). However, it is impossible to know what bands correspond to SPD-2 because none of them indicate SPD-2 molecular weight (91.5kDa). To solve this question, I compared protein extracts from N2 worms to those from adult hermaphrodites worms fed with E. coli expressing spd-2 dsRNA. Using spd-2 (RNAi), I could determine, by observing any missing bands, which one(s) correspond(s) to SPD-2. However, my results were not convincing (Figure 7, lane 4). Indeed, I do not observe any differences between protein extracts from spd-2 (RNAi) animals and those from wild-type worms. Therefore, I have not been able to conclude anything yet. Modification of Cdk-targeted phosphorylation site in SPD-2 sequence Another way to determine wether Cdk-phosphorylation affects SPD-2 activity in centriole disassembly is to modify Threonine 233, the most probable Cdk-pphosphorylation site on SPD-2, by an amino acid that cannot be phosphorylated. The substitution of Threonine 233 by a Valine (Threonine and Valine differ only by the presence of a kinase-targeted hydroxyl group on Threonine lateral chain) was performed by high throughput site-directed mutagenesis. A PCR performed with a mutagenic oligonucleotide primer generated a mutated codon (ACT into GTA, Gehin Charlotte Master 1 ENS LYON according the genetic code) in the spd-2 genomic sequence from pMR811. Then, the plasmid was bombarded into living unc-119 (ed3) mutant worms (viable and uncoordinated) to allow the integration of a few copies of the mutated spd-2 transgene into the genome. Only unc-119 (ed3) animals transformed with the plasmid containing the wild-type unc-119 gene survive starvation (on the contrary, unc-119(ed3) cannot survive dauer formation) while non-transformed animals and those that had lost unstable arrays do not survive .Then, modifications on the mutated SPD-2::GFP and any location changes during centriole elimination can be analyzed as previously described (fluorescence imaging and Western Blot). However, this experiment is still underway. DISCUSSION & PERSPECTIVES 1. DISCUSSION What mechanism governs the centriole elimination during oogenesis? It was previously demonstrated that SPD-2 acts upstream of centriole assembly (3, 18) but when centrioles disappear during oogenesis, SPD-2 is not eliminated at the same time (7, 8). Therefore, we can wonder why it is not immediately degraded and what happens. In this study, I have demonstrated that SPD-2::GFP protein shifts from the cytoplasm to the pachytene nuclei during centriole elimination in wild-type hermaphrodite C. elegans. In order to show the change of behavior of SPD-2, I used the fluorescence imaging but I had to overcome some technical difficulties. Firstly, I tried to detect SPD-2-GFP either in living adult pie-1::GFP::spd-2 worms or in dissected and fixed gonads (DAPI staining), using a compound microscope. However, the signal was too weak and there was a strong background, probably because of the small size of centrosome and of the autofluorescence that is usually observed in living worms. Then, I performed immunofluorescence to label SPD-2 in N2. But I could not obtain satisfying pictures since background fluorescence level was too elevated. Only staining SPD-2::GFP transgenic animals with monoclonal antibodies against GFP led to convincing results. Yet, it was previously described that an immunofluorescence assay in female germ line with the same antibodies against SPD-2 than those used in this study, did not give as much noise, if it was analyzed by confocal microscopy (8). A confocal microscope consists, basically, of a high quality compound microscope with a laser illuminator, electronic image detector and computer for image storage and processing, with an adjustable confocal pin hole in the imaging plane. Hence, confocal microscope allows a high-resolution epi-fluorescence microscopy, the acquisition of thin optical sections of finite, but controlled thickness and the decrease of the background fluorescence level of the samples. Thus, a more precise analysis with confocal Gehin Charlotte Master 1 ENS LYON microscope may confirm the presence of SPD-2 in the late pachytene of N2 germ lines, using antibodies against SPD-2 instead. Is SPD-2 modified during oogenesis in wild-type worms? Is the location change of SPD-2 during centriole elimination linked to physical modifications of the protein? In order to discover if SPD-2 could be modified during oogenesis, I performed Western Blots to identify the presence different forms of denatured SPD-2 protein at different stages of C. elegans development with antibodies against SPD-2. I evidenced several bands that conflict with the predicted size of SPD-2. Therefore, I then performed a Western blot comparison between proteins from spd-2(RNAi) worms (10) with proteins from N2. However, contrary to what I expected, no band disappeared in spd-2 (RNAi) animals (while spd-2(RNAi) should have prevented the traduction of SPD-2 and the missing band should have indicated what band corresponds to SPD-2). Having used spd-2 (RNAi) by feeding and knowing this method produces slightly more variable results than RNAi by soaking or injection (9), I am currently performing again western blot with new spd-2 (RNAi) worms. Other troubleshooting can reduced the observed background: on one hand, apply the antibody to the sample once or twice may reduce the unspecific binding; on the other hand, since it is possible that repetitive uses of protein samples generate proteolysis, it is necessary to use fresh protein extract. Finally, no firm conclusion on modifications of SPD-2 during post-embryonic development can be drawn from my analysis. However, it was demonstrated that there are Cdk-phosphorylation sites within SPD-2 sequence contains and that the most probable of them are conserved in other nematodes (12). So, if it is expected that SPD-2 can be regulated by Cdk-phosphorylation, a more precise phosphorylation analysis of SPD-2 could be performed by Mass spectrometry. A Large-scale identification of C. elegans proteins by multidimensional liquid chromatography-tandem mass spectrometry performed by a Japanese team, in 2003 (14) has demonstrated that among the approximately 5,400 peptides assigned in this study, many peptides with post-translational modifications, such as N-terminal acetylation and phosphorylation, were detected. Indeed, the phosphorylation of SPD-2 may have already been characterized and it would be interesting to obtain an English version of their article. 2. PERSPECTIVES Does Cyclin E/CDK2 target SPD-2 or other centriolar components? If it is soon demonstrated that SPD-2 can be phosphorylated and that this phosphorylation corresponds to centriole elimination, it will be then necessary to demonstrate if SPD-2 is a target of cyclin E/Cdk2. That SPD-2 is a target of Cdk2 suggests a physical interaction between the two Gehin Charlotte Master 1 ENS LYON proteins. In order to confirm this physical interaction, it would be interesting to use reverse proteomic approaches, like the yeast two-hybrid system (9). However, if this method shows that the complex cyclin E/Cdk2 is not responsible for the phosphorylation of SPD-2, it would be interesting to investigate which kinase activity is involved in SPD-2 phosphorylation. In the C. elegans embryo, loss of spd-2 gene activity results in a failure of centrosome duplication. As a consequence, bipolar spindles are not assembled; DNA is not segregated, cytokinesis fails and the embryos die (15). To identify factors that interact with spd-2 to regulate centrosome duplication, a sensitive genetic suppressor screen could be designed to identify mutations that restore normal centrosome duplication to an embryo deficient in spd-2 activity, as it was previously done to highlight factors which interacts with ZYG-1, an other kinase involved in centriole assembly (16). Among these suppressor mutations, we could detect gene encoding for kinases and then, investigate more precisely how these kinases physically interact with SPD-2, by the yeast two-hybrid system, as well. To know if cyclin E/Cdk2 target other centriolar components, it would be necessary to do again the same experiments than for SPD-2. However, another solution could be the highlighting of Cdk2 protein complex in C. elegans. A Tandem affinity purification (TAP), performed with the tagged-Cdk2 could bait its targets among C. elegans proteins and these proteins would be then analyzed by mass spectrometry. Is SPD-2 modified to regulate its localization? Another question rises from the observation of SPD-2 shift from cytoplasm to nucleus: Is a post-transcriptional modification of SPD-2 responsible for this shift and consequently for centriole disassembly or on the contrary, is this probable modification responsible for centriole disassembly and indirectly for SPD-2 sending in nucleus? To answer, it would be interesting to lead epistasis genetic experiments with RNAi against each component of centriole assembly (spd-2, zyg-1, sas-4, sas-5 and sas-6) in order to determine how centriolar proteins act the ones compared to others during centriole elimination in the germline, for instance, as it was precedently made to determine sequential protein recruitment in C. elegans centriole formation (3, 18). Thus, we would be able to know on the one hand, if SPD-2 acts upstream centriole disassembly; on the other hand, if localization change of SPD-2 results from a direct modification of the coiled-coil protein or from the modification of one of these partners, like another component of centriole assembly. That is why Cdk-phosphorylation analysis is currently performed in every known components of centriole assembly: ZYG-1, SAS-4, SAS-5 and SAS-6. Gehin Charlotte Master 1 ENS LYON On the other hand, as it was recently demonstrated for ZYG-1 (16), a genetic suppressor screen could highlight the presence of genes encoding for nuclear envelope components among SPD-2 partners. That could explain the presence of SPD-2 inside nucleus during oogenesis or intestinal cells development. Do the same mechanisms apply both to the germline and intestine cells? Shift of SPD-2 coiled-coil protein is visible both in germline and intestine cells but SPD-2 is present as foci in late pachytene nuclei and seems to be diffused in intestinal nuclei. So, we can wonder, since these two kinds of cells undergo different cell cycle changes (meiosis and endoreplicative cycle, respectively), if SPD-2 shift results from the same mechanism in both cases. Investigation of SPD-2 partners could help to the comprehension of each phenomena. Then, on the one hand, colocalization of each partner with SPD-2 by immunofluorescence experiments, with confocal microscope, on the other hand, experiments investigating the interaction between SPD-2 and Cyclin E/Cdk2, could lead to establish models of mechanism in each case. Gehin Charlotte Master 1 ENS LYON MATERIAL & METHODS Nematodes strains The following C. elegans strains were used: N2 Bristol was used as the wild type throughout. MR1163 (unc119 (ed3) III; [spd-2::spd-2::GFP; unc-119(+)], from Roy lab); OC92 ([pCK6.1: pie1::GFP::spd-2; unc119(+)] , Kemp et al. 2004); unc-119 (ed3); WH163 (nDf29/unc-13(e1091) spd-2(oj29) I), O'Connel et al. 1998). All C. elegans strains were cultured using standard techniques and maintained at 15°C unless stated otherwise (Brenner, 1974). Immunofluorescence microscopy For Immunofluorescence labeling, worms were dissected onto gelatin coated microscope slides in 10l PBST (PBS containing 0.1% Tween-20). They were then freeze-cracked in dry ice for 10 min before fixing in methanol at -20C for 1 min and post-fixing in formaldehyde solution for 30 min at room temperature. Washing slides three times for 3 min in PBST blocked non-specific sites. Primary antibodies (either rabbit polyclonal anti- SPD-2, a gift from K. O’Connell, National Institutes of Health, Bethesda MD or mouse monoclonal anti- GFP) were applied at 1 g/ml in PBST overnight at 4°C before washing 3 times for 10 min in PBST. Secondary antibodies (polyclonal anti-rabbit/mouse) were applied at 1 g/ml in PBST for 90 min at RT; the samples were washed as above before mounting. DAPI was applied at 0.7 g/ml for 1 min, before washing the slide in PBST for 1 min and adding 8 l Vector shield® on the slide. Indirect immunofluorescence microscopy was performed using a 60× oil-immersion objective lens in a compound microscope (DMR; Leica) equipped with a digital camera (C4742-95; Hamamatsu), imaging a μm-thick optical section. Images were processed using Photoshop 8.0 (Adobe). All microscopic works were performed at 20°C. Western Blot assay Protein extraction: For protein extraction, worms were immersed into H20 in presence of 5X protein loading buffer, before shifting the temperature (from -80°C for 15 min, to 95°C for 5 min) five times. The samples were then centrifuged at 13,500 rpm for 4 min and 5μl from the supernatant were used as protein extract. Gehin Charlotte Master 1 ENS LYON Western Blot: Each protein extract was dissociated on SDS-PAGE (10%) and transferred onto nitrocellulose membrane for 1 hour at 100V. Nitrocellulose membranes were then firstly incubated in appropriate dilutions of primary rabbit polyclonal antibodies (anti SPD-2, at 1:2000 or anti GFP, at 1:1000 in TBS 1X containing 5% milk) and primary mouse polyclonal antibodies (anti tubulin,) for 4h and then with secondary antibodies (Alkaline Phosphatase-conjugated antirabbit/mouse antibodies) for 1h. Chromogenic reaction was performed with Alkaline phosphatase (AP) staining kit (Sigma). Fluorescence was finally analysed by scanner machine. High-throughput in vitro site-directed mutagenesis (GeneTailor™ Site-directed Mutagenesis System, Invitrogen™) Target Plasmid: pMR811 is a 8 kb plasmid made from the pSK vector which contains the spd-2 gene promoter :: spd-2 genomic DNA sequence without the STOP codon and the last intron. 2.4 kb upstream of the ATG of the spd-2 gene was selected as the promoter. Control plasmid: The control plasmid is a 3.4 kb plasmid containing the lacZα gene, that when introduced into wild type E. coli produces blue colonies on plates containing LB/Amp/X-gal. 1) Methylation Reaction: 125ng of target plasmid DNA where methylated with DNA methylase for 1 hour at 37°C. 2) Amplification: The plasmid was amplified in a PCR mutagenesis reaction containing H20 (up to 50l) Phusion™ HF Buffer (1x), dNTPs (200M each), primers (0,5M each), target or control DNA plasmid template (12.5ng) and the Phusion™ High-Fidelity DNA polymerase (0,02U/l) Cycle Step Temperature (°C) Time Cycles Initial denaturation 98 30 s 1 Denaturation 98 8s Annealing 74/ 722 20 s Extension 72 3 min/ 45 s2 72 8 min 4 hold Final extension 1 2 Best efficiency for mutagenesis PCR. PCR cycling program for control plasmid and control primers. 201 1 Gehin Charlotte Master 1 ENS LYON Two overlapping primers (one of which contains the target mutation), designed according to Invitrogen™ Instruction manual: Primer sequences Tm (°C) 3 5'-ATTCCACAAATCACGACGAGAAAACGAGCGTACCTAAAAGAC-3' 4 79.60 5'- CTCGTCGTATTTGTGGAATTCATCGGTGA-3' 4 74.70 5'-GACCATGATTACGCCAAGCTTATAAATTAACCCT -3' 5 71,90 5'-AGCTTGGCGTAATCATGGTCATAGCTGTTT -3' 5 73,20 3 Tm calculation was made using the nearest-neighbor method, on www.finnzymes.com 4 Overlapping primers, red: overlapping region (20nt); black: extended region (10nt); green: mutation site 5 Control primers used with control plasmid and supplied with the GeneTailor™ mutagenesis System: The forward (mutagenic) primer contains a two-base substitution that introduces a Hind III site and stop codon within the lacZα gene. The stop codon generates a truncated LacZα protein and produces white colonies on plates containing X-gal 3) Transformation: The linear double-stranded DNA product containing the mutation was transformed into wild-type E.coli. The host cell circularized the linear mutated DNA and the McrBC endonuclease present in the host cells, digested the methylated template DNA, leaving only the unmethylated, mutated product. RNAi: As described previously (10), spd-2 RNAi was performed by feeding L1 worms with Escherichia coli expressing spd-2 dsRNA. Group-based phosphorylation scoring (GPS) method: http://bioinformatics.lcd-ustc.org/gps_web/predict.php (12) Gene bombardment: The method was adapted from the protocol "DNA transformation by gene bombardment" which is available on the website www.wormbook.org BIBLIOGRAPHY 1. Delattre M. and Gonczy P. (2004) The arithmetic of centrosome biogenesis. Journal of Cell Science.117; 1619-1629. 2. Boveri T. (1900). Zellen-Studien: Ueber die Natur der Centrosomen. Fisher, Jena, Germany. 220 pp elegans. Nature. 434; 462–469. Gehin Charlotte Master 1 ENS LYON 3. Pelletier L. and al. (2006) Centriole assembly in C.elegans. Nature. 444 4. Leidel S. and Gonczy P. (2005) Centrosome duplication and nematodes: recent insights from an old relationship. Dev. Cell. 9; 317-325. 5. Marshall WF. (2007) What is the function of centrioles? Journal of cell biochemistry. 100; 916-922. 6. Erich A. Nigg. (2007) Centrosome duplication: of rules and licenses. Trends in Cell Biology. 17; No.5. 7. Dae Young Kim and Richard Roy. (2006) Cell cycle regulators control centrosome elimination during oogenesis in C. elegans. Journal of Cell Biology. 174; No 6; 751-757. 8. Catherine A. Kemp and al. (2004) Centrosome Maturation and Duplication in C. elegans require the Coiled-Coil Protein SPD-2. Developmental Cell. 6; Issue 6; 511-523. 9. www.wormbook.org 10. Ravi S. Kamath and Julie Ahringer. (2003) Genome-wide RNAi screening in Caenorhabditis elegans Methods; 30, Issue 4, 313-321 11. Yu Xue, Fengfeng Zhou, Minjie Zhu, Guoliang Chen, and Xuebiao Yao. (2005) GPS: a comprehensive www server for phosphorylation sites prediction. Nucleic Acids Res. 1 ; 33(Web Server issue):W184-7. 12. Pelletier L. et al. (2004) The Caenorhabditis elegans Centrosomal Protein SPD-2 Is Required for both Pericentriolar Material Recruitment and Centriole Duplication. Current Biology. 14 ; 10, 863-873. 13. www.wormbase.org 14. Mawuenyega KG, Kaji H, Yamuchi Y, Shinkawa T, Saito H, Taoka M, Takahashi N, Isobe T (2003) Large-scale identification of Caenorhabditis elegans proteins by multidimensional liquid chromatography-tandem mass spectrometry. J Proteome Res.. 2(1):23-35. 15. 0'Connel KF. Et al.: (1998). A genetic screen for temperature-sensitive cell-division mutants of Caenorhabditis elegans. Genetics 149: 1303-1321 16. Kemp C. et al. (2007) Suppressors of zyg-1 define regulators of centrosome duplication and nuclear association in C. elegans. Genetics 176: 95-113 17. Harris JE. et al. (2006) Major sperm protein signaling promotes oocyte microtubule reorganization prior to fertilization in Caenorhabditis elegans. Developmental biology 299: issue 1; 105-121 18. Delattre M., Canard C. annd Gönczy P. (2006) Sequential protein recruitment in C.elegans centriole formation. Current biology 16: issue 18; 1844-1849 Gehin Charlotte Master 1 ENS LYON ANNEXES Figure 8. The most probable Cdk-phosphorylation sites of SAS-4, SAS-5, SAS-6, ZYG-1 and SPD-2 sequences, as determined by the Group-based Phosphorylation Site (GPS) scoring method (12). GPS scoring is a statistical method. Best scores correspond to most probable phosphorylation sites. On the figure, small stars surmount the amino acids most likely to be targeted by CDKs. Only scores higher than 4 were considered as significant. Figure 9. Centrioles are gradually lost after karyokinesis in intestinal nuclei. Extruded intestine from N2, stained for SPD-2 (green) and another centriole marker, SAS-6 (red) SPD-2 and SAS-6 colocalize in centriolar foci (stars) around nuclei (arrowheads) from the first larval stages (L1-L2). However, this number of foci decreases throughout post-embryonic development (as shown in the table) Gehin Charlotte Master 1 ENS LYON