CELL METABOLISM
advertisement

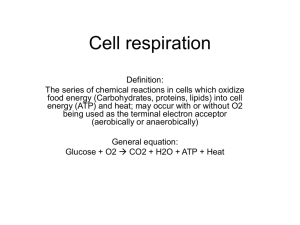
Biol 2424 Human Phys 1 CELLULAR PHYSIOLOGY Remember you are responsible for knowing the fx & structure of the cell & its organelles. I will not cover Ch 3 in lecture— most is review. DNA REPLICATION & PROTEIN SYNTHESIS Central Dogma: Genetic information flows in a one way direction from the nucleus of a cell to the cytoplasm. Genetic info is used to produce proteins from which almost everything else in the cell is made (by way of rxs). Diagram: Protein Synthesis Outline 1. Transcription takes place in the nucleus. “Waitress MiRNA: May I take your order please?” Helix separates (helicases), copy DNA onto mRNA (single strand). It carries enough info to make a polypeptide. mRNA carries the message out to the cytoplasm where translation takes place. 2. Translation takes place in the cytoplasm. “Cooks tiRNA & Ribby get the ingredients & make the protein meal.” - The message on the mRNA is translated by transfer tRNA's & ribosomes. Ribosomes are on the endoplasmic reticulum or rough ER - mRNA binds to the ribosome to act as the template for the tRNA that are carrying the amino acids that will build the new polypeptide or protein - Each tRNA carries a specific amino acid and temporarily binds to the ribosome. The tRNA’s form a chain, binding next to the previous tRNA; then the amino acids that each carries bind together; a series of polypeptides keeps growing as more & more tRNA’s bind and then release after their amino acids bond together in a peptide bond. - the polypeptide chains of amino acids (or proteins if longer 100 amino acids) are exported or for use in the cell itself. Where is the protein sent to be packaged for export? CELLULAR METABOLISM 1. A cell requires energy to assemble & disassemble the molecules it needs. 2. Where does this energy come from to power the cell? This energy comes into the cell in the form of bond energy, holding food molecules together. The cell extracts the energy from these bonds by a process described below, but the place where energy is extracted, the mitochondrion organelle, is not necessarily where the energy is ultimately needed. 3. To move energy around inside the cell, we use a kind of molecular rechargeable battery called ATP. To get the energy to provide for cellular functions we break the bonds in ATP. Remember energy is released when bonds are broken. Biol 2424 Human Phys 2 Diagram: ATP Cycles ATP–ADP CYCLE 1. First ATP bonds are broken, so that we are left with ADP + Pi, which releases energy; however, most of the energy stays with the broken-off phosphate, which we can then bond to the molecule that needs the energy. The process of adding a phosphate is phosphorylation. 2. The ADP molecule can then drift back to the mitochondrion and pick up another phosphate, which is then bonded to the ADP, using energy acquired from a food molecule (glucose). 3. The recharged ATP is released into the cell and used by a chemical rx that needs it. 4. How does the ATP know where to go in the cell to do the cell’s work? It doesn't have to "know." There is such a high density of them (tens of millions) diffusing throughout the cell that, unlike police officers, there’s always one around when you need one. You need about 10 million ATP's per second. If you didn't recycle ADP, then you’d have to manufacture about 120lbs, about 50kg, of ATP per day! 5. Where does the ATP come from? What we have is a cycle, in which energy comes into the body in the form of large food molecules and is converted through many processes to ATP: - In the digestive system these large food molecules are broken down into smaller ones, absorbed into the body, & transported to cells. - Once inside a cell, the small food molecules are almost completely broken down. - The energy released in this process is used to fasten a phosphate to an ADP molecule, making ATP. This cycling of ATP maintains a high concentration, so that there’s always enough bumping into compounds that “require energy to do their work.” 6. How do we get from food to ATP? Through cellular respiration. CELL RESPIRATION AND METABOLISM We are going to examine how animals capture and use the energy contained in chemical bonds to produce ATP & ADP. All carbohydrates get broken down into monosaccharides, which get absorbed into the body (facilitated diffusion) in the duodenum. They then get transported & absorbed into a cell. Biol 2424 Human Phys 3 POINTS TO KEEP IN MIND 1. These rxs don't occur in just one kind of cell or organ, they occur in just about every cell in the body. Right at this very instant, for example, in your integument cells, in your cardiac cells, pulmonary cells, everywhere—all the rxs I'm talking about are happening millions of times a second. 2. The following is a very simplified version of what happens. Just the high points to give you an idea of what's going on. For every step I cover, there are about 10-30 intermediate steps. The complete process of breaking down glucose in a cell to extract the energy is hundreds of steps. Why so many chemical steps? After all, you can break sucrose down in one step. One can burn a pure sucrose cube to get CO2, H2O, ashes, & heat. Why doesn't the body use one step? METABOLISM Metabolism is the process of extracting energy from various chemical rxs. It is the sum total of all chemical rxs of the body. The energy obtaining rx is to burn glucose: C6H12O6 + 6O2 36ATP + 6CO2 + 6H2O + heat Anabolism = chemical rxs that combine simple substance into more complex substances; require energy. amino acids proteins fats phospholipids monosaccharides polysaccharides Catabolism = chemical rxs that break down complex substances into simple ones & release energy. To release the energy you break the terminal phosphate group: ATP Adenosine~P~P + Pi+ energy The chemical rxs of metabolism require enzymes to proceed at an acceptable rate. Define enzyme: Diagram: Summary of Anabolism & Catabolism 4 Biol 2424 Human Phys OXIDATION/REDUCTION RXS These are the most important rxs in energy extraction & energy transfer in cells. 1. Reduction refers to the addition of an e- to a molecule, which is equivalent to the loss of H+ (a proton) from a molecule. - When you gain electrons, the charge of the molecule is reduced. 2. Oxidation is the removal electrons. This is equivalent to the addition of H+ to a molecule. “OIL RIG” Oxidation is Lose electrons & Reduction is Gain electrons. “LEO goes GERrrrr!” Lose electrons oxidation, gain electrons rrrrrreduction. 3. NADH NAD+ NAD+ is oxidized; it went from a charge of 0 to +1, so it lost e-. 4. NAD+ NADH NADH is reduced; it went from a charge of +1 to 0, so it gained an e-. 5. Oxidation/Reduction rxs are always coupled. When a substance is oxidized another is reduced. - Oxidation is usually energy releasing: The released energy is used to form ATP - Adenosine~P~P oxidation ATP = oxidative phosphorylation. (a squiggle represents a high energy bond) - Molecules with many hydrogen atoms are considered to be reduced and have the potential to undergo oxidation. CARBOHYDRATE METABOLISM 1. Carbohydrates in the diet consist of polysaccharides & disaccharides that get broken down during digestion into monosaccharides (glucose, fructose, galactose, mannose) 2. These are all absorbed in the small intestines (villi, microvilli, blood) and sent to liver. If the monosaccharide is not glucose, the liver converts into glucose, or shunts it into the metabolic (glycolytic) pathway in a different way. HOW DO YOU OBTAIN & USE ATP? Glucose is a cell’s main energy source. All the trillions of cells need it to do their job. Starches and other polysaccharides are broken down into monosaccharides in the small intestine. What happens to glucose once it’s absorbed depends on the cell’s own energy needs. ingestion digestion absorption distribution oral cavity, etc. stomach & sm. intestine villi microvilli capillaries/lymph vessels VvAa ECF ICF 1. If cells need immediate energy, glucose is oxidized by the cells. 2. If not needed by cells, it’s converted to glycogen (animal starch), the main storage form of glucose. Glycogen is formed in the liver through glycogenesis. Glycogen is in all cells, but primarily stored in the liver & skeletal muscle cells. Once the liver’s store is used up, cells break down lipids & proteins to make glucose. It’s estimated that the liver has a 4 hour store of glucose for the body. 3. If glycogen storage is adequate, the liver transforms glucose to triglycerides for storage in adipose cells through lipogenesis. 4. When cells need more energy, glycogen & triglycerides can be converted to glucose by glycogenolysis or gluconeogenesis. In gluconeogenesis, the final steps to glucose occur in the kidney or liver. 5. The glucose level in the blood is the blood sugar level (BSL) or blood glucose level, expressed in mg%; normal is 60-140mg%. HORMONES CONTROLING BLOOD GLUCOSE LEVELS Two pancreas hormones are the chief regulators of blood glucose levels 1. Glucagon produced by alpha cells in the pancreas, increase blood glucose. - promotes conversion of glycogen in liver to glucose = glycogenolysis - converts other nutrients to glucose (other than starches) = gluconeogenesis. - stimulates the release of glucose from the liver. - Secretion of glucagon is controlled by blood glucose levels by a negative feedback system. If you decrease BSL sensors in pancreas detect stimulates production of glucagon cells no longer stimulated in pancreas no glucagon produced. 5 Biol 2424 Human Phys 2. Insulin produced by beta cells in the pancreas, decrease B.G. levels -controlled by BSL by a negative feedback system. - increase BSL sensors in pancreas detect stimulates prod. of insulin decreases BSL pancreas cells no longer stimulated no insulin 3. Insulin has other fxs - aids transport of glucose from blood to cells facilitated diffusion - causes conversion of glucose to glycogen in the liver = glycogenesis - causes conversion of glucose to fatty acids lipogenesis - decreases glycogenolysis (glycogen to glucose) - decreases gluconeogenesis (other nutrients other than carbs into glucose) - also aids the entrance of amino acids into cells & the production of cellular protein GLUCOSE CATABOLISM 1. The oxidation of glucose is also known as cellular respiration—use glucose to make ATP. C6H12O6 + 6O2 + 6H2O 12H2O + 6CO2 + 36ATP + heat - Occurs in almost all the body’s cells & happens millions of times a second in every cell. - Provides the primary source of energy for cells. 2. The complete oxidation of one glucose molecule yields 30-36 molecules of ATP which occurs in 3 stages: 1. Glycolysis 2. The Krebs/Citric Acid Cycle 3. The Electron Transport Chain GLYCOLYSIS The first step in the process of obtaining ATP's is called glycolysis (= "break apart sweet") The chemical rxs that convert 1 molecule of glucose (6C) in to 2 molecules of pyruvate (pyruvic acid, 3C); it involves lots of enzyme catalyzed steps. 1. Glycolysis occurs in the cytoplasm of the cell. 2. Occurs without oxygen = anaerobic respiration 3. Use 2 ATP's to get the rxs going, produces 4 ATP’s, so net gain of 2 ATP's. 4. Only 3% of the maximum amount of energy made available by anaerobic respiration of glucose. What occurs next depends on the availability of oxygen. Pyruvate Lactic Acid in Mitochondrion ANAEROBIC RESPIRATION 1. Normally, muscles operate by aerobic (oxidative) respiration (i.e., oxygen available), but there are times when the demand for energy release is greater than the capacity of the circulatory system to bring oxygen to the muscle cells— there’s a shortage of oxygen. How does regular exercise help the muscle’s oxygen budget? 2. Normally, if a higher organism's cell runs out of oxygen, it shuts down its operation. But there is a kind of "emergencies only" metabolic pathway that lets you get a little energy out of glucose, even without oxygen. 3. What you do is combine pyruvate & NADH (nicotinamide adenine dinucleotide, reduced form) and convert it to lactic acid. 4. Glycolysis can proceed under anaerobic conditions (without O2). This capability is important in tissues, such as skeletal muscle when the demand for ATP rapidly increases at a time when oxygen is in low supply, such as in strenuous exercise. 5. The skeletal muscle cells are able to meet their increased requirement for ATP by increasing the rate of glycolysis and producing ATP, despite the fact that little or no oxygen is available. However, under these anaerobic conditions, the pyruvate produced by glycolysis is converted to lactic acid, which accumulates in the muscle, because it cannot be metabolized further. Lactic acid tends to be toxic to cells, and if too much builds up, the muscle will stop functioning. Have the problems of ATP depletion, creatine phosphate, torn connective tissue, etc. This is the point where you just can't do that last bench press, no matter how hard you try! Biol 2424 Human Phys 6 6. Lactic acid is transferred to the liver, where it is converted back to pyruvate and broken down by aerobic metabolism to CO2 & H2O. This requires additional oxygen, which is obtained by continued excessive breathing after muscular activity has ceased, a phenomenon known as oxygen debt. AEROBIC RESPIRATION Pyruvate is sent to mitochondria where it is completely oxidized during the KREBS CYCLE & the Electron Transport Chain. Normally all cells operate this way. If you run a marathon, or jog for that matter, you use the oxygen you inhale to operate in these aerobic metabolic pathways to provide the energy your muscles need to contract. Krebs Cycle (named for Sir Hans Krebs) is a series of oxidation/reduction rxs that occur in mitochondria of cells in the presence of adequate oxygen. - pyruvate is produced by glycolysis in the cytoplasm & is transported to the matrix of a mitochondrion. - pyruvate (3C) is converted to acetic acid. Coenzyme A (derived from vit B) is needed to facilitate the rx; coenzyme A & pyruvate are converted to acetyl CoA (2C), which enters the Krebs / Citric Acid cycle and bonds to oxaloacetic acid (4C). The citric acid cycle is like a stream wheel dumping out water to turn the mill grinder—it turns out the work horse ATP in its never ending rotation of rxs. Diagram: Pyruvate to Mitochondrion for Oxidation Consequences of the Series of Rxs in the Krebs Cycle 1. 2C removed (citric acid converted back to oxaloacetic acid to “recycle"). 2. Released C’s converted to 2 molecules of CO2 blood transports lungs exhaled from body. Oxygen in the CO2 comes from the original glucose molecule, not inhaled oxygen. Oxygen we breathe allows electron transport to occur (i.e. O2 is the final e- acceptor in the ETC). 3. Hydrogen atoms removed from molecules are capture by 2 hydrogen carrier nucleotide molecules NAD (vit B niacin derivative) & FAD (riboflavin vit B derivative). addition of electrons/H: NAD NADH = reduction FAD FADH2 = reduction 4. You get one molecule of ATP for each acetyl CoA processed. You have 2 acetyl CoA's/glucose molecule so you produce 2 ATP's for the Krebs cycle. 5. Total so far is 4 ATP's: 2 ATP’s from Glycolysis & 2 from the Krebs cycle. Importance of Krebs Cycle: - not the ATP produced, but the transfer of H to NAD (NADH) & FAD (FADH2), so they can enter the protein electron transport chain. - These nucleotides are excellent for carrying energy & then transferring it in the oxidative phosphorylation of ADP to make ATP; hydrogen atoms in NADH & FADH2 contain high energy e-‘s. Biol 2424 Human Phys 7 The Protein Electron Transport Chain (ETC) is the BIG PAYOFF!! Millions of winners every second!!! There is a considerable amount of energy still trapped in the released hydrogen atoms (electrons at high energy levels). Electron Transport occurs on the membrane lining the mitochondrial cristae (“folds, crests”). 1. Electrons extracted from NADH & FADH2 are transferred to electron carrier molecules called cytochromes. NAD & FAD can then be recycled to pick up more H atoms. 2. High energy electrons are sent through a series of rxs. Electrons lose energy at each step. 3. Electrons ultimately passed to oxygen derived from inhaled air (i.e. O2 = final e- acceptor). 4. Oxygen, which has gained electrons, combines with H+ (lost electrons) to form H2O. 5. Energy released by electrons is captured by ATP synthase. 6. ATP synthase converts ADP & Phosphate into ATP. By going through a series of electron transport rxs at least 32 ATP molecules are obtained for each glucose that’s oxidized. This is only 43% of the energy stored in the bonds of glucose; the rest is released as heat. Entropy can not be escaped—though life is highly ordered, it does not break the second law of thermodynamics. 7. Because oxygen is used, & phosphate added to ADP, it is called oxidative phosphorylation. If there’s no O2 (from the blood [or myoglobin in muscles]) as the final electron acceptor, aerobic metabolism stops. 8. Overall rx for oxidation of glucose is: C6H12O6 + O2 CO2 + H2O + ATP + heat GET 30-36 ATP's total: 28-32 from Electron Transport, 2 from Glycolysis, & 2 from Krebs Cycle Diagram: Overall Schematic of Cellular Respiration Biol 2424 Human Phys 8 ENERGY USAGE The energy stored in ATP is used to do work in the cell. Once formed, ATP is transported out of the mitochondrion and then is available as an energy source as needed within the cell. Three Categories of Energy (ATP) Use 1. synthesis of new compounds - protein synthesis - cell growth: some cells, especially those in a growth phase or ones with a high rate of secretion, use up 75% of the ATP they generate just to make new compounds 2. membrane transport – get materials into/out of the cell 3. mechanical work, such as muscle contraction SUMMARY - The chemical energy locked up in food molecules is broken down by digestive processes. - Controlled oxidation/reduction rxs accomplished by glycolysis, the Krebs cycle, & electron transport produce ATP. Some energy was spent to get more energy! It takes money to make money! - Most of the energy is released as heat, which is used to maintain a fairly constant body T and excess is eliminated to the environment. Why does a baby have brown fat? - Aerobic exercise allows cells to make enough ATP to perform work. Anaerobic pathway is used in “emergencies” (speed work, such as sprinting). - The more work one does, the more heat generated. LIPID METABOLISM 1. Lipids are the primary fuel storage biomolecules 2. 2nd to sugars as an energy source, but yield the greatest energy per unit weight. 3. Triglycerides stored in adipose tissue are the body's last energy reserve - can store more triglycerides than glycogen - more difficult to catabolize 4. Before triglycerides can be metabolized for energy they must be split by lipases into glycerol & fatty acids = lipolysis Diagram: Triglyceride Structure 5. Glycerol is converted to glyceraldehyde-3-phosphate, a compound formed during the catabolism of glucose, so here is where it enters glycolysis, and is then broken down into pyruvate. 6. Fatty acids are oxidized in mitochondria and many are converted to Acetyl CoA. Lipids contain 9 kcal of energy; whereas proteins & carbohydrates have 4 kcal. 7. The liver has to oxidize fatty acids differently (it can’t convert 2C units into acetyl CoA); liver oxidizes fatty acids to ketone bodies. These acids can seriously disrupt the body’s pH. Ketone bodies generally found in small #'s (body prefers glucose). When ketone bodies rise above normal = ketosis (acidic blood). 8. lipid anabolism (lipogenesis): liver cells & fat cells can synthesize lipids from glucose or amino acids - occurs when excess lipids (CHO) enter body (too many to be used now or stored as glycogen). - CHO triglycerides or lipoproteins or cholesterol - excess protein amino acids triglycerides Biol 2424 Human Phys 9 PROTEIN METABOLISM During digestion proteins are broken down to amino acids, which can be used for energy, but are mainly used to synthesize new proteins for growth, repair, hormones, & enzymes. Protein Catabolism (Holy Catastrophic Calamity, Batman!) - some proteins catabolized, even if energy needs are adequate, so as to make new proteins. - liver converts proteins to triglycerides, glucose, and CO2 & H2O. - liver has to remove nitrogen from amino acids; it converts amino acids into organic acids such as Acetyl CoA, pyruvate, & other intermediates that are used in aerobic metabolism. Where is Acetyl CoA used? - when liver removes N from amino groups, NH3 forms, which quickly reacts to become NH4+; ammonia & ammonium are toxic, so the liver converts them to urea (CH4N2O), which the kidney excretes. Urea is the main nitrogenous waste in mammals; many other vertebrates excrete the pasty uric acid (see car or statue). Protein Anabolism/Synthesis (see above) - formation of new proteins by joining amino acids together - occurs in the cytoplasm by mRNA & tRNA binding to ribosomes in a particular order as directed by DNA.