Module 1: What Should We Do About Global Warming?
advertisement

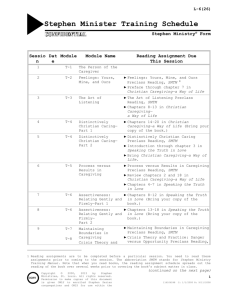
Chemistry 149 Syllabus Fall 2010 Instructor: Dr. Laura Stultz SSC-340 (x4877) email: lstultz@bsc.edu Office Hours: M 1:00-3:00pm; Tu, Th 1:00-3:30pm other times by appointment if I am in my office with the door open, you are welcome to drop in Text: Silberberg; Chemistry: The Molecular Nature of Matter and Change 5th Edition ChemConnections Modules Global Warming Computer Chip Thermochemistry Water Treatment Course goals and content: This is a challenging one-semester general chemistry course for students with an excellent high school background in science. We will be learning chemistry in the context of topics related to the environment and technology. We will not only discuss the science, but how it is applied in real world problems and how political and economic considerations also come into play. This course is based on class discussion, group problems solving, and discovery based laboratories. You will make extensive use of materials on the worldwide web and a CD-ROM included with your modules. You also will be required to attend talks during the common hour by outside speakers. Consistent with the aims of 1Y courses this course will emphasize: reading and writing skills – research papers, laboratory reports, reading scientific literature oral communication – oral presentations, debates, class discussion technology use – use of scientific instruments, accessing information on the web data analysis and interpretation – designing laboratory experiments and interpreting data collected by others collaborative learning – group problem solving and laboratory work the impact of science on our society – global and local environmental issues The course is divided into three modules: Module 1: What Should We Do About Global Warming? Topics covered : Electromagnetic Spectrum and Spectroscopy, Chemical Equations, Stoichiometry, Lewis Structures, VSEPR Theory and Molecular Shape Module 2: Computer Chip Thermochemistry: How Can We Create An Integrated Circuit From Sand? Topics covered: Thermochemistry, Properties of Metals, Nonmetals, and Metalloids, First Law of Thermodynamics, Calorimetry, Second Law of Thermodynamics, Third Law of Thermodynamics, Bond Enthalpies, Gibbs Free Energy Function Module 3: Water Treatment Topics covered: Solubility, Dynamic Equilibrium, Le Chatelier’s Principle, Acid/Base Chemistry, Buffers, CASPiE project on anti-oxidants Grading: You have a pre-class assignment for each class. The assignment will either be collected or incorporated into a brief quiz or writing assignment at the beginning of class. Each Wednesday you will be given a problem set that is due the following Wednesday. Late class assignments or problem sets will not be accepted unless you have an excused absence. Each module has a quiz and a graded project. Laboratory notebooks will also be graded each week. At the end of the semester you will complete an independent laboratory project. An exam will be given at the end of each module. The final exam will be comprehensive. The student must make arrangements with the instructor for taking quizzes/exams early if the student knows he/she will be absent (i.e., school function, athletic trip, ect.). Late exams will only be permitted for documented emergency absences. If you will be missing a class due to illness, you need to contact me via voicemail (x4877) or email before the beginning of class and you must have a doctor's excuse before you will be permitted to make up any work. The instructor makes the final determination of the validity of the absence. Your final grade will be computed using the following point distribution: Daily assignments/labs/problem sets (10 points each) 200 points Module quizzes (25 points each) 75 points Global Warming Debate 50 points Computer Chip Lab Report 75 points Antioxidant Lab Report 150 points Module Exams (100 points each) 300 points Final Exam 150 points Total 1000 points The above distribution of points is approximate may be subject to minor changes. Honor Code: Much of the work in this class will be collaborative in nature so it is expected that you will discuss assignments with your classmates. However, when you turn in work as your own, you need to be sure that what you have written is your understanding of the concept and not simply copied from another student’s work. If you are using another student’s laboratory data, you need to be sure to document and reference the data in any written laboratory report and your laboratory notebook. Take home quizzes or tests should not be discussed with anyone until they are turned in to the instructor. If you are taking a quiz or exam early for any reason, you should not discuss any aspect of the quiz or test with your classmates. If you are found in violation of the Honor Code by the Honor Council, you will receive no credit for the assignment, test, or quiz in question. Module 1: What Should We Do About Global Warming? Outline Wednesday Sept 1: Class introduction Session 1: What Do We Need to Know About Global Warming? In class: go over syllabus; Read and discuss articles on global warming. Introduce culminating project. Lab 1: Wed Sept 1 An Inconvenient Truth Video and discussion. Debate groups will be assigned. Friday Sept. 3 Meet in Debate Groups/ Articles on Global Warming Preclass assignment: Finish reading Exploration 1A and answer "Gathering Information" and “Working with Information” questions 1-5 on pages 2-5. Summarize two different scientific articles on global warming. These articles must include actual data supporting or refuting the theory of global warming. This data is likely to be displayed in the form of a graph, but might also be given in tabular form. Each summary should be approximately one page; include a copy of the graphical data as well. The key idea for this assignment is to explain how the data supports the argument in the article. Popular journals such as Time magazine and the New York Times science page have published good review articles that do include some data. Be sure to cite the sources of your articles. Wednesday Sept. 8: Session 1: What Do We Need to Know About Global Warming? Exploration 3A: How Are The Atoms In Greenhouse Gas Molecules Connected? Preclass Assigment: Review Section 10.1 in text pages 378-388. In class: Review Lewis structures, formal charge, and resonance. Complete Lewis structures in table on page 23. Lab 2: Wed Sept 8: Exploration 5A: Is there CO2 in your breath? Session 6: What are Your Personal Contributions to Greenhouse Gas Emissions? In class: Discuss sources and sinks. Perform experiment in Exploration 5A Part I and answer questions 1-2 in your lab notebook. Perform Exploration 6A and 6B recording all calculations in notebook. Friday Sept. 10: Exploration 3B: What Are the Shapes of the Greenhouse Gas Molecules? Preclass Assignment: In textbook Read sections 10.2-10.3 on pages 388-401. Do problems 10.6, 10.8, 10.14, 10.16, at the end of chapter 10. In class: Violations of octet rule. Build models and complete chart on page 23. Handout Problem Set 1 due in one week. Monday Sept. 13: Exploration 3C: What Determines Whether A Gas Absorbs Infrared Radiation? Preclass Assignment: Complete problems 10.12, 10.20,10.24 10.43, 10.45 in text book In class: Discuss polarity and dipole moments; IR tutor. Wednesday Sept 15 Handout: Molecular Modeling and NMR Preclass Assignment: If you have any difficulties see me on Tuesday in my office. Read handout on NMR. Complete the Pre-Class Assignment on page 8. In class: Meet in the Organic Lab SSC 317. Use molecular modeling software to look at 3-D images of molecules. Begin Parts 1-3 in handout. NMR spectra of unknown compounds will be run. Lab 3: Wed Sept 15 Handout: Molecular Modeling and NMR Prelab Assignment: Complete the table in the Pre-lab exercise on pages 12-13. In lab: Meet computer lab on 2nd floor. Complete Parts 1-2 that were not completed in class. Each group will analyze the unknown spectra that were run this morning. As a class you will match the unknown spectra to the compounds shown in the pre-lab assignment. Friday Sept. 17: Hybridization and Valence Bond Theory Preclass Assignment: Problem Set 1 due at the beginning of class. Read Sections 11.1 -11.2 on pages 411-421 in your textbook Complete problems: 10.56, 10.58, 10.62, 10.63, 10.71, 10.85, 10.94 In class: Discuss hybrid orbitals and valence bond theory. Work problems together in class. Hand out Problem Set 2 due in one week. Monday Sept. 20: Debate preparation. Preclass Assignment: Have data and outline of debate strategy. Your groups should have a draft of your opening statement. In class: Review Module material and debate format. Work in groups on your questions and strategy. Wednesday Sept. 22: Preparation for Final Project and Quiz 1 Preclass Assignment: Prepare for Quiz 1. In class: Final discussion of issues. Quiz 1 in class. Wed Sept 22: Final Project – Debate on Global Warming To be done during laboratory period. Friday Sept 24 Module Review Preclass Assignment: Problem Set 2 due. In class: Review module material, quiz 1 and problem set 2. Monday September 27: Module 1 exam One hour in class exam (75%) and take home portion (25%). Take home exam due Wednesday at the beginning of class. Module 2 Computer Chip Thermochemistry: How Can We Create An Integrated Circuit From Sand? Wednesday September 29: Session 1: Introduction Session 2: Why Doesn’t Elemental Si Exist In Nature? In class: Discuss conductors, insulators, components of integrated circuits; heat, work, and internal energy. Wed September 29 Lab 1: Session 4: How Can We Reduce the Heat Required To Extract Si? Preclass Assignment: Read Exploration 4B-4D in your module In Class: Discuss calorimetry, specific heat, and heat capacity. Perform experiments in Explorations 4B-4D. Record data and experimental procedure in notebook and answer questions assigned by instructor. Friday October 1: Exploration 2D: What Evidence Do We Have For Conservation of Energy? Exploration 3A: The Story-Line Preclass Assignment: Using the movies and information at the site http://wwnorton.com/college/chemistry/chemconnections/ answer questions 1-9 in section 1B of your and questions 1-7 in section 1C in your module to be turned in. You may want to try the Self-Assessment quizzes after each section to test your understanding of the material. In your textbook read sections 61 and 6.2 pages 236-245. In class: Discuss the First Law of Thermodynamics, exothermic and endothermic reactions. Complete Exploration 2C, 2D and 3A. Problem Set 3 will be handed out to be turned in next Friday October 8. Monday October 4: Exploration 4A: The Story Line Preclass Assignment: Read Exploration 3B and answer questions 1-6. Read Exploration 3C and answer questions 1-5. Bring textbook to class. In class: Discuss homework. Discuss Hess’s Law and do problems from text in groups. Wednesday October 6: Exploration 5A: The Story Line Exploration 5B: In Which Direction Do Chemical Systems Become More Disordered. Exploration 5C: How Do We Calculate Entropy Changes? Exploration 5D: Why Do Systems Tend Towards Disorder? Preclass Assignment: Read Exploration 5A and answer questions 1-7. If you would like a more detailed discussion of entropy see sections 20.1 and 20.2 in your textbook In class: Discuss spontaneous reactions, entropy and the Second Law of Thermodynamics. Complete Exploration 5B. Discuss the Second and Third Law of Themodyanmics, standard enthalpies, and microstates. Complete Explorations 5C and 5D in groups. Wednesday October 6 Lab 2: Project 9B: Finding A Viable Sequence Of Tasks To Produce A Complex Pattern On a Metal Substrate – Part 1 Preclass Assignment: Read Creating the context for Project 9B on pages 98-99. Complete the table on page 99 under the Before Laboratory Period 1 section. You will need to use thermodynamic data in Appendix 9B on pages 107-109. In class: We will begin our two week final laboratory project, Project 9B. In groups we will complete the section Laboratory Period 1 and combine our data to prepare for the second part of the project to be completed next week. Plan a strategy for designing your own computer chip for the next lab. Friday October 8: Session 6: Gibbs Free Energy and Spontaneity Preclass Assignment: Problem Set 3 due at the beginning of class. In class: Discuss how H, S, and temperature effect spontaneity and the role of G. Complete Exploration 6A. Begin Exploration 6B and go over Enthalpy vs. Entropy Graphing Tool and class assignment due Monday. Problem Set 4 will be handed out and is due next Monday October 18. Monday October 11: Session 7: Extent of Reaction; Preclass Assignment: Using the Enthalpy vs. Entropy Graphing Tool at the Chem Connections website ( http://wwnorton.com/college/chemistry/chemconnections/), complete questions 4-11 on pages 64-65 in Exploration 6B. In class: Discuss standard conditions and the difference between G and G. Complete Exploration 7A. . Wednesday October 13: Session 8: Extent of Reaction under Non-standard Conditions; Quiz 2 Preclass Assignment: Prepare for Quiz In class: Discuss questions from Exploration 7C. Introduce Extent of Reaction Simulator. Quiz 2 in class. Monday October 18: Session 8 Bond Enthalpy Preclass Assignment: Problem Set 4 due at the beginning of class. In class: Discuss bond enthalpies. Complete Explorations 8A and 8B. Begin Exploration 8C if time permits. Wednesday October 20: Final Wrap up Preclass Assignment: Complete questions 1-6 on page 92 in Exploration 8C if they were not completed in class. In your textbook complete problems 9.49, 9.83, 9.89 on pages 374-376. In class: Discuss problem set and any follow up questions. Wednesday October 20 Lab 3: Project 9B: Finding A Viable Sequence Of Tasks To Produce A Complex Pattern On a Metal Substrate – Part 2 Preclass Assignment: Review data from last weeks lab and read Before Laboratory Period 2 on pages 101-103. Consider the two possible methods given and consider if both would work and which would be preferable. Design a procedure of your own. In class: Use your procedure to produce your model transistor. Postclass Assignment: You will write a written laboratory report, detailing your procedure and defending your choice of process. This will be due electronically Friday November 5 by 5:00pm. Friday October 22: Module 2 Exam. One hour in class portion and a take-home portion that is due Monday October 25 at the beginning of class. Module 3: Water Treatment: How Can We Purify Our Water?/Antioxidants in Foods Monday October 25: Session 1: Introduction In class: Discuss CASPiE project and role of antioxidants in food. Wednesday October 27: Session 4: Equilibrium Preclass Assignment: Work on lab preparations and journal article study guide due Friday. In class: Discuss dynamic equilibrium, and equilibrium expressions. Complete Explorations 4A, 4D in class. Discuss equilibrium simulator in Exploration 4C. Wednesday October 27 Lab 1: Determination of ascorbic acid in lemon juice. Preclass Assignment: Read introduction to CASPiE Module and the week 1 experiment. In you lab notebook prepare the procedure for the titration experiment. In class: Determine the amount of ascorbic acid in fresh and concentrated lemon juice using iodine titration and ascorbic acid standards. Friday October 29: Journal article analysis Preclass Assignment: Read article by Arts et.al. and complete journal article study guide. In class: Discuss article in class and go over questions in study guide. Monday November 1: Exploration 4E: How can we use equilibrium expression to predict equilibrium concentrations? Preclass Assignment: Using the equilibrium simulator at the ChemConnections website complete Exploration 4C and turn in questions 1-5 on pages 77-79. In class: Discuss results from reaction simulator. Discuss solubility product constants, and molar solubility. Complete Explorations 4E. Wednesday November 3: Exploration 4F: How can you tell whether a reaction has reached equilibrium? Preclass Assignment: Complete Questions 1-7 on pages 83-85 in your module. In your textbook complete questions 19.71, 19.73, and 19.79 on page 875. You will need information from Appendix C to complete some of these problems. In class: Discuss reaction quotients. Complete Problems 1-14 in Exploration 4F. Review equilibrium problems. In class work examples from chapter 17. Wednesday November 3 Lab 2: Total Polyphenolic Assays Preclass Assignment: Read Laboratory 2 for the Total Polyphenolics Assay. Calculate the amounts of reagents you will need to make your solutions and how you will perform your dilutions. Write introduction and procedure up in your laboratory notebook. In class: In groups you will make up solutions for total polyphenolic assays. Perform total polyphenolic assay on tea sample and standard solutions. Friday November 5: Section 17.5 in Text: Calculating equilibrium concentrations Preclass Assignment: In your textbook complete problems 17.36, 17.37, 19.75, 19.87 19.89 Computer Chip Lab report due at 5:00pm In class: Bring textbook to class. Discuss methods for solving equilibrium problems. Work examples from chapter 17. Handout Problem Set 5 due in one week. Monday November 8: Section 17.6 in Text: Le Chatelier’s Principle Preclass Assignment: Do problems 17.48, 17.50, 17.52, 17.54, 17.90 at the end of chapter 17. In your textbook read section 17.6 pages 745-753. In class: Discuss Le Chatelier’s Principle. Work example problems in class and demonstrate reversible reactions. Wednesday November 10: Acids and Bases Preclass Assignment In your textbook read sections 18.1-18.3. Do problems 17.64, 17.68, 17.72, 17.75 In class: Discuss, acids and bases, strengths of acids and bases, conjugate acid/base pairs, Ka, Kb, Kw, pH. Complete Explorations 7B and 7C. Wednesday November 10 Lab 3: TEAC assay Preclass Assignment: Read Laboratory 3 in the CASPiE Module and prepare an introduction and procedure in your lab notebook In class: Measure TEAC activity of trolox, tea and epicatechin. Calculate the trolox equivalents of tea sample. Friday November 12: Exploration 7D: How do you determine the equilibrium concentrations of strong and weak acids and bases? Preclass Assignment: Problem Set 5 due at beginning of class. In class: Discuss weak acid/base equilibria. In class work problems in Exploration 7D and problems from chapter 18. Handout Problem Set 6 due Monday November 22. Monday November 15: More weak acid/base problems Preclass Assignment: Read sections 18.4-18.5 in textbook pages 782-793. Do problems 18.10, 18.24, 18.30, 18.34, 18.50. Turn in draft for introduction of anti-oxidant paper. In class: Further discuss weak acid/base equilibria. In class work problems from chapter 18. Introduction to acid/base properties of salts. Wednesday November 17: Module 3 Quiz Section 18.7 Acid-Base Properties of Salts Preclass Assignment: Prepare for Quiz. In your textbook read sections 18.7 on pages 796-799 In class: Discuss hydrolysis of salts and acid-base titrations. Work through problems in textbook in class. Wednesday November 17: Final Lab Projects Preclass Assignment: In your notebook prepare your procedure for your independent project on antioxidants in tea. If you need an additive for the tea (milk, honey, lemon), bring it to lab unless I tell you that we have it available. Friday November 19: Section 19.1 Equilibria of Acid-Base Buffer Systems Preclass Assignment: In your textbook do problems 18.68, 18.76, 18.99, 18.101, 18.103. In class: Discuss Buffer solutions. Work several problems in class Monday November 22 Exploration 4G: How is Free Energy Related to the Extent of a Reaction. Preclass Assignment: Problem Set 6 due at the beginning of class. In class: Discuss how ∆G, ∆G°, K and Q are related in describing the extent of a reaction. Complete questions 1-6 on pages 90-92 of module. This material is also covered in sections 20.4 in your text book. Monday November 29: Final Module review Preclass Assignment: In your textbook complete problems 19.28, 19.30, 20.77, 20.79, 20.81, In class: Work on buffer worksheet if needed and review for exam. Wednesday December 1: Module 3 Exam One-hour in class exam with take-home portion due Friday December 3 Wednesday December 1: Complete Research project. Paper due Tuesday December 7. Friday December 3: Take Home exam due at beginning of class. Discuss kinetics and mechanism Monday December 6: Discuss kinetics and mechanism. Do textbook problems in class. Tuesday December 7 : Final lab report due electronically by 11 pm. Wednesday December 15: Final Exam 9:00am- noon