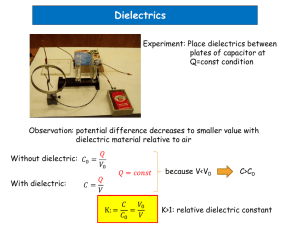
MRS PROCEEDINGS TEMPLATE
advertisement

The Effect of UV treatment on Mechanical and Dielectric Properties of Ultra-low Dielectric Materials Bo-Ra Shin, Kyu-Yoon Choi and Hee-Woo Rhee* Department of Chemical and Biomolecular Engineering, Sogang University, 1 Shinsu-Dong, Mapo-gu, Seoul 121-742, Korea ABSTRACT In this study, we prepared nanoporous dielectric films using organosilicate matrix and chemically reactive porogens. The organosilicate matrix was copolymer of methyl trimethoxysilane (MTMS) and 1,2-bis(triethoxysilyl) ethane (BTESE), and the porogen was trimethoxysilyl xylitol (TMSXT). UV light of 254 and 352 nm wavelengths with 7 mW/cm2 intensity was irradiated during thermal curing on the nanoporous dielectrics for various times. This, as a result, improved the dielectric as well as mechanical properties. The elastic modulus of UV-cured sample increased to 10.7 GPa at k= 2.16. This is because network structures and Q structures formed by increased network structure with decreased cage structure. UV irradiation also broke Si-CH3 groups of MTMS, which made more free volume into dielectrics at the same porogen loading. Therefore, UV treatment did not only show the increased porosity but also decreased dielectric constant in spite of the formation of denser network structures. INTRODUCTION The main issues in non-memory devices such as microprocessor unit (MPU) and systemon-chip (SoC) are high speed of data processing and low power consumption in addition to high integration density. Owing to continuous shrinkage of the device and requirement for higher performance, new low k materials are urgently required. One of the promising candidates is methyl silsesquioxane (MSSQ) oligomers. The MSSQ materials have less polar methyl groups, because they have lower dielectric constant (k ~ 2.7) than SiO2 (k ~ 4). In addition, ultra-low-k dielectrics are being developed by the introduction of nano-sized pores into existing low-k materials, which is the most effective way to reduce the dielectric constant. However, the pores cause abrupt reduction in mechanical properties such as elastic modulus and hardness above certain porosity. Thus, the appropriate nanoporous ultra-low dielectric materials having lower dielectric constant and stronger mechanical properties are being developed as a promising candidate. However, both of the properties are incompatible. In particular, the pores, which were introduced to reduce the dielectric constant, affect the mechanical properties contrastingly. For mechanical enhancement of the nanoporous dielectrics, the film was treated by UV light which was one of the most effective methods because it has chemical and structural effects on the nanoporous dielectrics by breakage and rearrangement of silicon bond structures. EXPERIMENTAL DETAILS The copolymer of methyltrimethoxy silane (MTMS, 75 mol%) and 1,2bis(trimethoxysilyl) ethane (BTESE, 25 mol%) were used as a matrix. Poly(methyl silsesquioxane) (PMSQ) based on MTMS has inherently low dielectric constant (k= 2.7) but mechanically fragile. BTESE increased the number of crosslink junctions per monomer, so elastic modulus increased with BTESE content. We chose 25 mol% BTESE copolymer whose dielectric constant is 2.9. Trimethoxysilyl xylitol (TMSXT) as a reactive porogen was prepared by allylation and hydrosilylation. For allylation, allylbromide was added dropwise into xylitol. For hydrosilylation, the allylated xylitol were dissolved in thrimethoxy silane with platinum oxide (PtO2) as a catalyst. The obtained hydrosilylated xylitol were purified to remove PtO2 by column chromatography using celite and activated carbon. The trimethylsilane end groups of porogens were expected to react with the copolymer matrix during thermal curing. The matrix and porogen were dissolved in 1-methoxy-2-propanol acetate (PMA) at 20 wt% and each solution was mixed with controlling the content of the matrix from 0 to 60 vol%. The mixed solution was spun on Si wafer at 2,500 rpm for 30 sec and the films were thermally cured at 250 ℃ for 2 hr, at 300 ℃ for 2 hr and at 430 ℃ for 1 hr. To make uniform pores and mechanically strong matrix, UV light of 254 and 352 nm wavelengths with 7 mW/cm2 intensity was irradiated during thermal curing at 430 ℃ on the nanoporous dielectrics for various times (5, 10 and 30 min). The dielectric constant of the films was measured by the LCR meter (Hewlett-Packard 4284A) at 1 MHz. Mechanical property was measured by a nanoindenter (Nano Indenter®XP, MTS Corp.) in the continuous stiffness measurement mode. In order to analyze effect of molecular structure (suboxide, network, cage) via UV treatment, solid-state NMR (unity INOVA, Varinan) and FT-IR (NICOLET, THERMO Fisher Scientific) were used. DISCUSSION 1H-NMR Spectra For minimization of phase separation of porogen and matrix, porogen, which had trimethoxysilyl [Si-(OCH3)3] group, was synthesized. Trimethoxysilyl [Si-(OCH3)3] group helps chemical combination between two phases. We synthesized these porogen (TMSXT) through allylation and hydrosilylation and checked the result by 1H-NMR Spectra. Trimethoxysilyl [Si(OCH3)3] group in terminal group of porogen materials was confirmed by SiCH2 peak in 0.6 ppm and Si-(OCH3)3 in 3.6 ppm. Figure 1. 1H-NMR spectra of TMSXT Analysis of Si-O-Si bonding structure To check effect of UV treatment on ultra low dielectric film after spin-coating, UV light of 254 and 352 nm was irradiated. The irradiation of 352 nm didn’t affect any bonding related to silcon atoms. In case of irradiating UV light in 254 nm, decrease of suboxide and cage structure was observed and the proportion of cage structure per total network structure also decreased compared to non UV treatment, even though network structure increased. That is, network structure was increased by decreasing suboxide group. It can be expected as Si-C bonding on one side was broken by joining Si-OH in the reaction with pyrolysis of methylene bridge and Si-CH3 bonding and SiO2 network were formed at the same time. The matrix had suboxide structure by ethylene bridge between Si atoms, which were caused by introduction of Si-CH3 of MTMS terminal group and BTESE. Because the increase of network structure was observed on 30 minute irradiation in 254 nm, we searched variation of structure change in each irradiation time. The more amount of matrix, the more effect happened in the shadow of UV irradiation. The reason was that the suboxide content, which was effected by UV light, decreased with decline of matrix. (a) (b) (c) Figure 2. Peak area ratio of nanoporous films upon UV exposure time (a) matrix (b) at 30% porogen loading (c) at 60% porogen loading Mechanical intensity Figure 3 show elastic modulus and hardness of UV-treated films. In case of dielectrics, which were treated with UV irradiation (254 nm, 10 min), elastic modulus and hardness increased by about 15%. It was regarded that the network structure ratio increased by the effect of Si-O-Si structure through UV irradiation. Thus, we used 29Si-NMR to search chemical and structural changes, and the increase of Q4 structure, perfect SiO2 structure, was confirmed from figure 4. Hence, Q3 and T structure showed a tendency of decreasing proportions. That meant the increase of mechanical property was caused by Q3 and T structure change as well as reduction of the cage structure. 3.0 non-UV 254nm 10min UV 16 14 12 10 8 non-UV 254nm 10min UV 2.5 Hardness (GPa) Elastic modulus (GPa) 18 2.0 1.5 1.0 6 0 30 Porogen loading (vol%) (a) 60 0.5 0 30 Porogen loading (vol%) (b) Figure 3. (a) Elastic modulus and (b) hardness of nanoporous ultra-low dielectrics 60 Figure 4. Changes of 29Si-NMR spectra and peak area ratio by UV irradiation Dielectric constant Generally, UV irradiation makes dielectric constants increased by improving curing density. However, porosity of dielectric film increased in this study, but dielectric constant also decreased. Therefore, to check section in which carbon contents decreased, 13C-NMR was used. Basically, the matrix consists of Si-CH3 structure from MTMS and Si-CH2-CH2-Si structure from BTESE. Each group was detected respectively by -3 and 5.6 ppm in 13C-NMR. Because peak area of Si-CH3 decreased with UV irradiation, it would be explained that UV treatment affected MTMS group of matrix and Si-CH3 was decomposed. Also, free volume in the film increased from decomposing Si-CH3 and dielectric constants was reduced by increased porosity. 35 Porosity (%) 25 20 15 10 5 Dielectric constant (k) 3.0 non-UV 254nm 10min UV 30 non-UV 254nm 10min UV 2.8 2.6 2.4 2.2 0 0 30 Porogen loading (vol%) (a) 60 0 30 Porogen loading (vol%) (b) Figure 5. (a) Porosity and (b) dielectric constant of nanoporous ultra-low dielectrics CONCLUSION 60 A final target of UV treatment on ultra-low dielectric materials base approach was to obtain the UV effect about structure and properties of ultra low dielectrics. The research was fulfilled by using 254 and 352 nm of UV wave. In general, in case of 254 nm, suboxide and cage structure proportions reduced and network proportion relatively increased. Thin films were exposed to 254 nm UV lamp for increasing treatment time (5, 10 and 30 min) and mechanical properties were increased with increasing treatment time. The largest enhancement was achieved when the thin film was treated for 5 min and small enhancements were achieved when the treatment time increased further. In addition, UV treatment effect was observed by Si-C bonding change, and the matrix, which had ethylene bridge between Si atoms, improved mechanical properties through increasing network structure. Moreover, Si-CH3 group of MTMS in matrix was decomposed and free volume was created. As a result, the UV treatment improved the dielectric and mechanical properties at once and its elastic modulus of UV-cured one showed 10.7 GPa at k= 2.16 (254 nm, 10 min). Accordingly, UV treatment resulted in decreased dielectric constant in spite of the denser network structures. REFERENCES 1. P. V. Zant, Microchip Fabrication: A Practical Guide to Semiconductor Processing, Mcgraw-Hill: New York, p 1 (2000). 2. M. Padovani, L. Riester, L. Rhodes, S. A. B. Allen and P. A. Kohl, J. Electrochem. Soc., 149, F171 (2002). 3. T. Cho, F. M. Pan, K. J. Chao, P. H. Liu and J. Y. Chen, Thin Solid Films, 483, 283 (2005). 4. Zenasni, B. Remiat, C. Waldfried, Ch. Le Cornec, V. Jousseaume and G. Passemard, Thin Solid Films, 516, 6 (2008). 5. Chun Hsien Huang, Hui Ling Huang, Chen I Hung, Na Fu Wang, Yeong Her Wang and Mau Phon Houng, J. Jpn. Appl. Phys., 47, 1532 (2008).