Universitetet i Oslo
advertisement
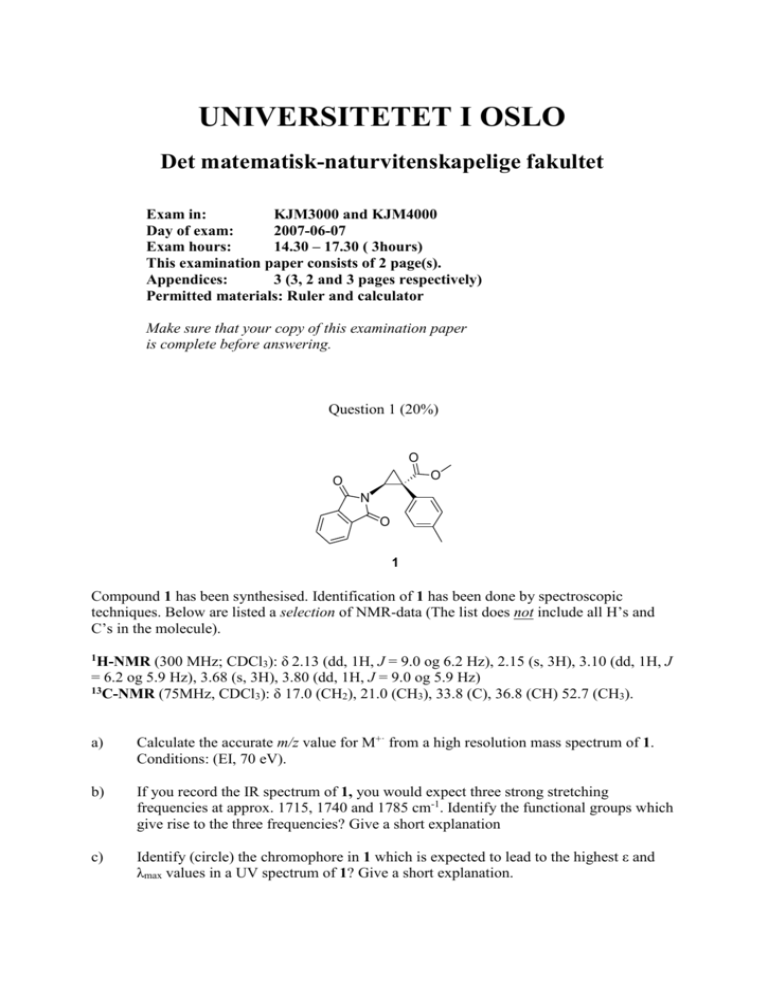
UNIVERSITETET I OSLO Det matematisk-naturvitenskapelige fakultet Exam in: KJM3000 and KJM4000 Day of exam: 2007-06-07 Exam hours: 14.30 – 17.30 ( 3hours) This examination paper consists of 2 page(s). Appendices: 3 (3, 2 and 3 pages respectively) Permitted materials: Ruler and calculator Make sure that your copy of this examination paper is complete before answering. Question 1 (20%) O O O N O 1 Compound 1 has been synthesised. Identification of 1 has been done by spectroscopic techniques. Below are listed a selection of NMR-data (The list does not include all H’s and C’s in the molecule). (300 MHz; CDCl3): δ 2.13 (dd, 1H, J = 9.0 og 6.2 Hz), 2.15 (s, 3H), 3.10 (dd, 1H, J = 6.2 og 5.9 Hz), 3.68 (s, 3H), 3.80 (dd, 1H, J = 9.0 og 5.9 Hz) 13C-NMR (75MHz, CDCl ): δ 17.0 (CH ), 21.0 (CH ), 33.8 (C), 36.8 (CH) 52.7 (CH ). 3 2 3 3 1H-NMR a) Calculate the accurate m/z value for M+· from a high resolution mass spectrum of 1. Conditions: (EI, 70 eV). b) If you record the IR spectrum of 1, you would expect three strong stretching frequencies at approx. 1715, 1740 and 1785 cm-1. Identify the functional groups which give rise to the three frequencies? Give a short explanation c) Identify (circle) the chromophore in 1 which is expected to lead to the highest ε and λmax values in a UV spectrum of 1? Give a short explanation. d) Assign the listed 1H-NMR-signals of 1 to the structure and give a short explanation of the coupling pattern. e) Assign the listed 13C-NMR-signals of 1 to the structure. Question 2 (40%) An “unknown” compound has been studied spectroscopically. Propose a molecular structure for the “unknown” compound based on the spectroscopic data found in attachment 2. Assign as many of the signals in the 13C- and 1H-NMR spectra as possible and give a brief explanation for your assignments. Draw equations to account for the fragmentation reactions which produce the indicated fragments. Question 3 (40%). An “unknown” compound has been studied spectroscopically. Propose a molecular structure for the “unknown” compound based on the spectroscopic data found in attachment 3. Assign as many of the signals in the 13C- and 1H-NMR spectra as possible and give a brief explanation for your assignments. Draw equations to account for the fragmentation reactions which produce the indicated fragments. A HRMS spectrum of the unknown gave m/z = 84.0577 ± 0.0005 for M+·. Briefly explain the difference between the two IR spectra (in attachment 3) in the area above 3000 cm-1. Vedlegg 1 / Attachment 1 Vedlegg 2 / Attachment 2 Grunnstoff analyse / Elemental analysis: C = 44.8 %, H = 3.27 % 1 H-NMR: 90 MHz, 42mg in 0.5 ml CDCl3 δ: 10.6 (1H, s), 7.44 (2H, d, J = 8 Hz), 7.15 (2H, d, J = 8 Hz), 3.58 (2H, s) 13 C-NMR: Broadband decoupled, 25MHz, 350 mg in 1.5 ml CDCl3 δ: 177.7, 132.1, 131.7, 131.1, 121.5, 40.4. IR: KBr disc. MS: EI, 75 eV. Vedlegg 3 / Attachment 3 Masseformel basert på nøyaktig masse fra de mest naturlig forekommende isotoper. Formula mass based on the accurate mass of the most abundant isotopes. 1 H-NMR: 400 MHz, 0.5 ml in 0.5 ml CDCl3 δ: 4.33 (1H, m), 3.53 (1H, s), 2.48 (1H, s), 1.78 – 1.70 (2H, m), 1.01 (3H, t) 13 C-NMR: Broadband decoupled, 15 MHz, 20 volume % in CDCl3 δ: 85.0, 72.9, 63.4, 30.7, 9.4. IR: Liquid film IR: Gas phase MS: EI, 70 eV