Introduction: P. aeruginosa and Acinetobacter species are
advertisement

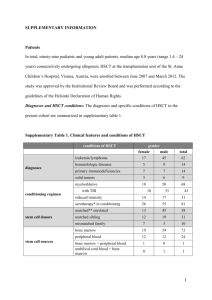
Introduction: P. aeruginosa and Acinetobacter species are organisms commonly causing nosocomial infections and often associated with a resistant antibiotic profile. Carbapenems are the the antibiotic of choice for treatment of infections caused by cephalosporin resistant gram negative bacilli due to their broad spectrum of activity and stability to hydrolysis by most beta-lactamases. This scenario is now changing with the emergence of metallobeta-lactamase (MBL) producing strains, especially among non fermenting Gramnegative bacilli (NFGNB) like P.aeruginosa and Acinetobacter species.[1, 2] Resistance to carbapenems is due to any one of the following mechanisms: decreased outer membrane permeability, increased efflux systems, alteration of penicillin binding proteins and production of carbapenem hydrolyzing enzymes (Carbapenemases). According to Bush, Jacoby and Medeiros molecular classification carbapenem hydrolyzing enzymes are classified into four groups A, B, C and D. MBLs belong to group B and are enzymes requiring divalent cations as cofactors for their activity. MBLs are inhibited by metal ion chelators such as CuCl3, FeCl3, EDTA, sodium mercaptoacetic acid (SMA), mercaptopropionic acid (2MPA) and 2-mercaptoethanol (2ME) but not by clavulanic acid, sulbactam or tazobactum. The MBLs efficiently hydrolyze all β-lactams, except aztreonam in vitro. [3] Thus aztreonam is the treatment of choice for infections caused by MBL producers. The IMP and VIM genes responsible for MBL production are horizontally transferable via plasmids and can rapidly spread to other bacteria. [4] Although there are various methods recommended for screening MBL production currently there are no guidelines recommended by the central laboratory standards institute (CLSI). Commonly used for detection of MBL production are Modified Hodge test (MHT) (DDST) [6], [5], imipenem/ceftazidime-EDTA double disc synergy Test impregnated imipenem (IMP) discs [7] and minimum four-fold reduction in MIC values with imipenem EDTA combination. [8] It is important to use simple and reliable tests for detection MBL production among clinical isolates of gram negative bacilli. This will help in appropriate treatment of patients especially in intensive care settings and to control the spread of antibiotic resistance. Therefore this study was undertaken to assess the local burden of MBL production among NFGNB and to evaluate three phenotypic tests used for MBL detection. Materials and methods: This study was undertaken in the Department of Microbiology, St.John’s Medical College, Bangalore from January to May 2009 during which a total of 8638 gram negative bacilli were isolated from various clinical samples such as wound exudates, blood, CSF, urine, body fluids, respiratory secretions etc. 742 (8.58%) of these were NFGNB. 116/742 (15.6%) of NFGNB were multi drug resistant. 100 consecutive non repetitive isolates of NFGNB resistant to imipenem (10µg) and ceftazidime (30µg) isolated from clinical samples of patients admitted to this tertiary care referral centre were included in the study. NFGNB were speciated using standard phenotypic and biochemical tests.[9] Antibiotic susceptibility was tested by Kirby Bauer disc diffusion method in accordance with CLSI guidelines, incorporating standard strain of P. aeruginosa ATCC 27853 and E.coli ATCC 25922 for quality control.[10,11] The antibiotics tested were piperacillin (100μg) gentamicin (10μg), amikacin (10μg), netilmicin (30μg), ciprofloxacin (10μg), cefoperazone (75μg), ceftazidime (30μg), carbapenems [imipenem (10μg), meropenem (10μg)], polymyxin B (300U), piperacillin/tazobactam (100/10μg) and aztreonam. MBL production was suspected when the isolate was resistant to ceftazidime (CZD) and/ or imipenem (inhibition zone diameter <18 mm for CZD and <16 mm for IMP). IMP resistance was confirmed by determining the MIC by agar dilution according to CLSI guidelines.[11] (IMP concentrations ranging from 0.062-128 μg/ml were tested). Isolates with imepenem MIC of <4 μg/ml and >16 μg/ml are considered to be sensitive and resistant respectively.[11] IMP resistant strains were screened for MBL production by MHT and IMP-EDTA disc synergy test. DDST was done according to Lee et. al IPM-EDTA disc method of Yong et al [7] [6] modification of by adding zinc sulphate to the medium to increase the sensitivity of the test. Migliavacca et. al. [8] reported the use of microdilution method for determining a fall in MIC values with IMP-EDTA combination, but we used agar dilution method for the same. Modified Hodge test (MHT): uses E.coli ATCC 25922 and a 10 μg IMP disc instead of S.aureus ATCC 25923 and a 10 μg penicillin disc used in Hodge test. [5,12] A lawn culture was made on Muller Hinton Agar (MHA) using overnight culture suspension of E. coli ATCC 25922. (opacity of the tube adjusted by comparing with a 1:10 dilution of 0.5 McFarland opacity standard). After brief drying, a 10 μg IMP disc was placed at the center of the plate and test strains were streaked from the edge of the disc to the periphery of the plate in four different directions. Following overnight incubation, MBL positive isolates showed a ‘clover leaf pattern’ in the zone of inhibition. IMP-EDTA DDST [6,7]: Three modifications of this test were evaluated for sensitivity in detection of MBL. An overnight broth culture of the test strain, (opacity adjusted to 0.5 McFarland opacity standard) was lawn cultured on a MHA plate. In the first modification a 10 μg IMP disc and a blank filter paper disc (6 mm diameter, Whatmann filter paper no. 2) were placed 2.5 cms apart centre to centre. 10 μl of 0.5 M EDTA (Sigma, USA) solution was added to the blank disc, (approximately 1.5 mg/disc). In the second modification IMP and EDTA discs were placed touching each other edge to edge. In the third modification two IMP discs 10 (µg) were placed on the surface of an inoculated MHA agar plate and 10 μl of 0.5 M EDTA solution was added to one of the discs. The zone of inhibition around both the IMP discs was recorded and compared following 16-18 h incubation at 350C. An increase in zone size of at least 7 mm around the IMP-EDTA disc is suggestive of MBL production. 0.5M EDTA solution was prepared by dissolving 186.1 g of disodium EDTA.2H2O in 1000 ml of distilled water and adjusting it to pH 8.0 by using NaOH. The mixture was sterilized by autoclaving. For long term usage EDTA impregnated IMP discs were dried in an incubator and stored at 4oC and -20oC in airtight vials without desiccant. MIC of imipenem EDTA combination[8]: MIC of imipenem EDTA combination was determined by agar dilution method. 1ml of EDTA solution was added to 1 ml of the imipenem solution spanning similar concentrations as done for MIC to imipenem alone. Each 2 ml of EDTA and imipenem in graded concentrations was added to 18 ml of molten MHA and poured on plates that were allowed to set. A fixed inoculum of the test strains was spot inoculated on the agar. The reading was taken after 18-24 h of incubation. Highest dilution inhibiting the growth of organisms was recorded as the MIC. Results: 100 consecutive NFGNB isolates resistant to IMP (10µg) and CAZ (30µg) by disc diffusion method were obtained from clinical samples. (One isolate per patient) as shown in Figure 1. 70% of the isolates were from critically ill patients admitted in medical and surgical intensive care units. 66 of these were P.aeruginosa and 34 were Acinetobacter species (Acinetobacter baumanii complex 30, Acinetobacter lwofii 4). Antibiotic resistance in these isolates was as follows: 100% resistance to piperacillin, gentamicin, CAZ, cefaperazone, imepenem and meropenem, amikacin (73%), netilmicin (75%), ciprofloxacin (88%), cotrimoxazole (96%), piperacillin/tazobactam (48%). All the isolates were sensitive to polymyxin B and aztreonam. Of the 100 NFGNB resistant to IMP by disc diffusion, 93 had MIC values in the resistant range (8-128 µg/ml) and seven showed intermediate resistance (MIC between 4-8 µg/ml). Results of MBL detection by the three phenotypic methods is summarized in Table 1. 55 isolates were MBL producers by MHT (Figure 2). In addition to the above 55 isolates IMP-EDTA DDST detected MBL production in an additional eighteen isolates (Figure 3). The three modifications of the DDST test detected MBL production in 65, 67 and 73 isolates respectively. 8-128 fold reduction of MIC with imipenem EDTA combination was observed in 74 isolates. P.aeruginosa ATCC 27853 and E.coli ATCC 25922 neither exhibited a zone size enhancement with EDTA nor a fall in MIC. Potency of EDTA impregnated IMP discs stored at 40C and -200C was checked every week. Discs stored at -200C retained potency upto 16 weeks. Discussion: MBL was first reported as a zinc dependent enzyme in Bacillus cereus in mid 1960s. [13] Few decades later, imipenem hydrolyzing metallo enzymes were found in Aeromonas hydrophila[14] and Bacteroides fragilis.[15] All these enzymes were produced by chromosomal genes and at first were recovered only from single clinical isolates. In 1991, Japan reported the first plasmid-mediated MBL in P. aeruginosa.[16] Apart from P.aeruginosa, MBL production was also seen in other bacteria such as Serratia, Klebsiella pneumoniae, Escherichia coli, Enterobacter aerogenes, Enterobacter cloacae, Citrobacter freudii, Proteus vulgaris and Acinetobacter.[17] In this study MHT detected MBL production in 55/100 imepenem resistant NFGNB. EDTA impregnated IMP disc was able to identify MBL production in 73 isolates (an additional 18 isolates). MIC to IMP-EDTA combination detected MBL production in 74 isolates. Studies across India report rates of MBL production ranging from 72%100% among carbapenem resistant NFGNB. The first report of MBL production in India was in 2002 from urban hospital in Bangalore[18] which reported MBL production in all the 6 (100%) carbapenem resistant P.aeruginosa. In 2005 Hemalatha V et al [19] reported MBL production among 87.5% of carbapenem resistant P.aeruginosa and all the MBL producers were resistant to fluoroquinolones and aminoglycosides. From south India Jesudasan et al[20] reported MBL production in 72% of imepenem resistant NFGNB with concurrent resistance to aminoglycosides like Gentamicin, amikacin and the quinolones which is similar to the findings in our study. Gupta V et al [21] reported MBL production in 86.11% of imepenem resistant NFGNB using imepenem EDTADDST method. Varaiya et al [22] recorded MBL production in 83.3% carbapenem resistant P.aeruginosa isolated from ICU patients with 100% resistance to aminoglycosides and cephalosporins. A study from rural India [23] reported imepenem resistance of 8.62% among P.aeruginosa and 93.3% of these were MBL producers with 100% resistance to all the antibiotics tested. This high rate of MBL production among P.aeruginosa in rural settings is alarming. In our study the rate of MBL production among carbapenem resistant NFGNB is 74%. The likely reasons for carbapenem resistance among non MBL producers be varied such as, decreased cell membrane permeability or activity of efflux pumps.[24] Predominant NFGNB showing imipenem resistance was P. aeruginosa, with majority being isolated from respiratory samples like BAL, tracheal trap and sputum of patients admitted in intensive care units. MBL producers in this study showed 100% resistance to piperacillin, Gentamicin, ceftazidime, cefaperazone and 48% were resistant to piperacillin- tazobactum which is lower than the findings of Navneeth et al [18] and Chaudhray et.al [25] who have reported 100 per cent resistance to piperacillin- tazobactum. However in this study all the MBL producers were sensitive to polymyxin B (300U) and aztreonam. Recently studies have shown that polymyxins are not as toxic as previously thought 26] and thus there is a renewed interest in the use of non- traditional agents including polymyxin B and E (colistin) for the treatment of serious infections caused by MDR P. aeruginosa. However monotherapy with these agents should be avoided and combination therapy with a sensitive aminoglycoside or fluroquinolone is preferred. In the absence of a novel antibiotic agent in the near future polymyxin B should be used judiciously. As CLSI does not provide guidance for susceptibility testing of polymyxins, serum bactericidal assays become prudent to regulate the dosage when these agents are used. There are reports on MBL production in P.aeruginosa from countries across the globe such as Brazil [27], Korea [28], Singapore [29] and France [30]. There are reports of MBL production in P. aeruginosa from the Asian and the Pacific countries, namely Hong Kong, Taiwan and Japan [31] as well. Although there are several screening methods used and recommended for the detection of MBL production, currently there are no CLSI guidelines for the detection of these enzymes. MHT is a simple test for screening for MBL production but occasional false negative results have been reported.[6] This could be avoided by incorporating zinc sulphate in a final concentration of 70μg/ml into the MHA.[6] In this study EDTA impregnated IMP disc showed a better sensitivity in the detection of MBL which is similar to the observations of other workers. [6, 20] 8-128 fold decrease in MIC values with imipenem EDTA combination was observed in this study as compared to a 4-512 fold reduction as reported by Migliavacca et al.[8] Other methods such as PCR [33] [32] and E test have been used to identify MBL producers. However, these tests may not be cost- effective for routine testing in clinical laboratories. PCR has become more difficult with the increasing number of types of MBLs.[7] Conclusion: For infections caused by MBL producing NFGNB therapeutic options are severely limited. Therefore identification of MBL producers is of great importance for the appropriate treatment of these infections and also to control the spread of resistance. Thus a simple screening test is essential to monitor resistance. The two screening tests for MBL detection namely, IMP-EDTA DDST and MIC to IMP-EDTA combination are equally effective. Based on the findings of this study we conclude that for MBL detection EDTA disk synergy test is more sensitive than MHT and much simpler than the MIC detection for imepenem EDTA combination. With the stored EDTA discs having a shelf life of upto 16 weeks this is a convenient method in a regular bacteriology laboratory to monitor the production of MBLs and for antibiotic resistance surveillance. However we could not include a standard ATCC carbapenem resistant positive control in the study since none is available for Nonfermenting gram negative bacilli and there is no standardized methodology recommended by CLSI. References: 1. Kahan FM, Kropp H, Sundelof JG, Birnbaum J. Thienamycin: Development of imipenen-cilastatin. J Antimicrob Agents Chemother 1983; 12 (Suppl D): S1-35. 2. Franklin C, Liolios L, Peleg AY. Phenotypic detection of carbapenem susceptible metallo ß-lactamase producing Gram negative bacilli in the clinical laboratory. J Clin Microbiol 2006; 44: 3139-44. 3. Bush K, Jacoby GA, Medeiros AA. A functional classification scheme for βlactamases and its correlation with molecular structure. Antimicrob Agents Chemother 1995; 39: 1211-33. 4. Bennett PM. Integrons and gene cassettes; a genetic construction kit for bacteria. Antimicrob Agents Chemother 1999; 43: 1-4. 5. Lee K, Chong Y, Shin HB, Kim YA, Yong D, Yum JH. Modified Hodge and EDTAdisk synergy tests to screen metallo-β-lactamase producing strains of Pseudomonas and Acinetobacter species. Clin Microbiol Infect 2001; 7: 88-91. 6. Lee K, Lim YS, Yong D, Yum JH, Chong Y. Evaluation of the Hodge test and the imipenem-EDTA double-disk synergy test for differentiating metallo-β-lactamase producing isolates of Pseudomonas spp. and Acinetobacter spp. J Clin Microbiol 2003; 41 : 4623-9. 7. Yong D, Lee K, Yum JH, Shin HB, Rossolini GM, Chong Y. Imipenem-EDTA disk method for differentiation of metallo-β-lactamase-producing clinical isolates of Pseudomonas spp. and Acinetobacter spp. J Clin Microbiol 2002; 40 : 3798-801. 8. Migliavacca R, Docquier JD, Mugnaioli C, Amicosante G, Daturi R, Lee K, et al. Simple micro dilution test for detection of metallo-β-lactamase production in Pseudomonas aeruginosa. J Clin Microbiol 2002; 40: 4388-90. 9. Koneman EW, Allen SD, Janda WM, Schreckenberger PC, Winn WC Jr. The non fermentative Gram negative bacilli. In: Color atlas and textbook of diagnostic microbiology, Lippincott 5th ed. Philadelphia; 1997 p. 293-303. 10. Bauer AW, Kirby WMM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disc method. Am J Clin Pathol 1996; 45: 493-6. 11. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; 15th Informational Supplement. CLSI/NCCLS M100- S18. Wayne (PA); 2008. 12. Hodge W, Ciak J, Tramont EC. Simple method for detection of penicillinaseproducing Neisseria gonorrohoeae. J Clin Microbiol 1978; 7: 102-3. 13. Sabbath LD, Abraham EP. Zinc as a cofactor for cephalosporinase from Bacillus cereus 569. Biochem J 1966; 98: 11C-3. 14. Shannon K, King A, Phillips I. Beta-lactamases with high activity against imipenem and Sch 34343 from Aeromonas hydrophila. J Antimicrob Chemother 1986; 17: 45-50. 15. Cuchural GJ Jr, Malamy MH, Tally FP. Beta-lactamase mediated imipenem resistance in Bacteroids fragilis. Antimicrob Agents Chemother 1986; 30: 645-8. 16. Watanabe M, Iyobe S, Inoue M, Mitsuhashi S. Transferable imipenem resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother 1991; 35 : 147-51. 17. Arakawa Y, Shibata N, Shibayama K, Kurokawa H, Yagi T, Fujiwara H, et al. Convenient test for screening Metallo-β-lactamase-producing Gram-negative bacteria by using thiol compounds. J Clin Microbiol 2000; 38: 40-3. 18. Navaneeth BV, Sridaran D, Sahay D, Belwadi MR. A preliminary study on metallo-β-lactamase producing Pseudomonas aeruginosa in hospitalized patients. Indian J Med Res 2002; 116: 264-7. 19. V Hemalatha, Uma Sekar, K Vijaylakshmi. Detection of metallo beta lactamase producing Pseudomonas aeruginosa in hospitalized patients. Indian J Med Res 2005; 122: 148-152. 20. Jesudason MV, Kandathil A.J., Balaji V. Comparison of two methods to detect carbapenemase & metallo-β- lactamase production in clinical isolates. Indian J Med Res 2005; 121: 780-783. 21. Gupta V.; Singla N.; Chander J. Use of two double disk synergy tests to detect metallo beta lactamases in non-fermenters. Indian J. Med. Res. 2008; 128:671672. 22. Varaiya A, Kulkarni N, Kulkarni M, Bhalekar P & Dogra J. Incidence of metallo beta lactamase producing Pseudomonas aeruginosa in ICU patients. Indian J Med Res 2008; 127: 398-402. 23. D.K. Mendiratta, Deotale V & Narang P. Metallo-β-lactamase producing Pseudomonas aeruginosa in a hospital from a rural area. Indian J Med Res 2005; 121: 701-703. 24. Buscher KH, Cullmann W, Dick W. Opferkwich W. Imipenem resistance in Pseudomonas aeruginosa resulting from diminished expression of an outer membrane protein. Antimicrob Agents Chemother 1987; 31: 703-8. 25. Chaudhary U.; Bhaskar H.; Sharma M. Imipenem-EDTA disk method for rapid identification of metallo-β-lactamase producing gram-negative bacteria. Indian J. Med. Res. 2008; 127:406-407. 26. Markou N, Apostolakos H, Koumoudiou C, Athanasiou M, Koutsoukou A, Alamanos I, et al. Intravenous colistin in the treatment of sepsis from multiresistant gram negative bacilli in critically ill patients. Crit Care 2003; 7: R78-83.) 27. Gales AC, Menezes LC, Silbert S, Sader HS. Disseminationin distinct Brazilian regions of an epidemic carbapenems resistant Pseudomonas aeruginosa producing SPM metallo-b-lactamase. J Antimicrob Chemother 2003; 52: 699702. 28. Lee K, Lim JB, Yum JH, Yong D, Chong Y, Kim JM, et al. blavim-2 cassettecontaining novel integrons in metallo-β-lactamse producing Pseudomonas aeruginosa and Pseudomonas putida isolates disseminated in a Korean hospital. Antimicrob Agents Chemother 2002; 46: 1053-8. 29. Koh TH, Wang GC, Sng LH. IMP-1 and a novel metallo-b-lactamse, VIM-6, in flurorescent pseudomonads isolated in Singapore. Antimicrob Agents Chemother 2004; 48 2334-6. 30. Poirel L, Naas T, Nicolas D, Collet L, Bellais S, Cavallo ID, et al. Characterization of VIM-2, a carbapenems hydrolyzing metallo-b-lactamase and its plasmid- and integron-borne gene from a Pseudomonas aeruginosa clinical isolate in France. Antimicrob Agents Chemother 2000; 44: 891-7. 31. Bell JM, Turnidge JD. Carbapenem resistance among hospitalized patients in the Asia-Pacific region (APACO): Report from the SENTRY Antimicrobial Surveillance program, 1998-2001. Abstract P715 in the 13th European Congress of Clinical Microbiology and Infectious Diseases;2003 32. Senda K, Arakawa Y, Ichiyama S, Nakashuma K, Ito H, Ohsuka S, et al. PCR detection of metallo- β-lactamases gene (bla IMP) in Gram-negative rods resistant to broad spectrum β-lactams. J Clin Microbiol 1996; 34: 2909-13. 33. Walsh TR, Bolmstrom A, Qwarnstrom A, Gales A. Evaluation of a new E test for detecting metallo-β- lactamase in routine clinical testing. J Clin Microbiol 2002; 40: 2755-9. Figure 1: Shows the clinical samples from which NFGNB included in the study were isolated. DISTRIBUTION OF ISOLATES FROM CLINICAL SAMPLES CLT 1 1 CLINICAL SAMPLES DRAIN/ASCITIC FLUID 3 1 URINE 3 BLOOD 4 1 WOUND SWAB 7 13 2 PUS 9 1 ET TIP 4 2 SPUTUM 6 1 9 TREACHEAL TRAP 0 P.aeruginosa A.baumanii 15 2 A.lwoffii 4 6 8 10 NO.OF SAMPLES 17 12 14 16 18 Figure 2: MBL production by modified Hodge test: Isolates A and B are MBL producers showing a clover leaf pattern. Isolates C and D do not produce MBL. Isolate D is ATCC P.aeruginosa used for quality control. Figure 3: MBL production using IMP-EDTA Double disk synergy test Table1: Results of the three phenotypic tests for MBL detection Test No. of isolates positive for MBL Sensitivity Specificity PPV† NPV‡ P.aeru Acineto ginosa -bacter spps. MIC with IMP +EDTA 49 25 100% 100% 100% 100% DDST 48 25 98.6% 100% 100% 96.42% MHT 36 19 74.32% 97.1% 98.21% 60% (Sensitivity and specificity in comparison to MIC) †PPV- positive predictive value, ‡ NPV- negative predictive value