Supplementary text
advertisement

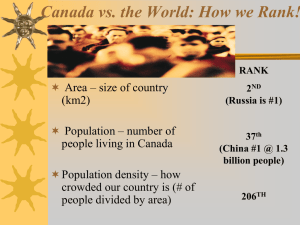
Appendix S1 Species’ traits predict the effects of disturbance and productivity on diversity Nick M. Haddad, Marcel Holyoak, Tawny M. Mata, Kendi F. Davies, Brett A. Melbourne, and Kim Preston Supplementary Material Methods for determining species traits Intrinsic growth rate, r, and carrying capacity, K We determined the intrinsic growth rate, r, and the carrying capacity, K, for each species at high and low nutrient levels as described in the main text. Figure S1 shows data from that experiment, including best fit models that support logistic growth. Estimates were generated for each of 5 replicates of high and low nutrient treatments. In each case, logistic growth was supported over exponential or theta-logistic growth. The final estimates of r and K were the average of the values generated from the 5 replicates. For one bottle of Paramecium bursaria and one bottle of Spirostomum ambiguum, we could not obtain an estimate. For high nutrient treatments used to predict disturbance responses in the main text, species differed in r (n=38, F = 68.16, p<0.001, with Colpidium sp. > Cyclidium sp. = P. aurelia > Euglena gracilis = S. ambiguum > Euplotes aediculatus = Coleps sp. = P. bursaria), and in ln(K) (n=38, F = 187.88, p<0.001, with E. gracilis > Cyclidium sp. > P. bursaria > Colpidium sp. = E. aediculatus = P. aurelia > Coleps sp > S. ambiguum). Haddad et al., Supplementary Material, Page 1 Dispersal rank The dispersal experiment was conducted in two 1-day blocks, using fresh multispecies communities on each day. To obtain multispecies communities, we mixed subcultures of individual species that were old enough to have reached their equilibrium density into one large culture. Communities contained 12 species each day, and among the 2 days included all 8 species that we considered in this paper. We homogenized and sampled this large culture twice to measure starting densities of individual species by counting the individuals of each species in ten weighed droplets. If at least 20 individuals of each species were not found in the original droplets, we continued to add more volume until we reached 20. Initial densities (#/ml) were, in Day 1: Coleps sp. = 1.2, Colpidium sp. = 2, Cyclidium sp. = 1210, Euglena gracilis = 6.5, Euplotes aediculatus = 0.933, Paramecium aurelia = 20; and in Day 2: Coleps sp. = 3.33, Colpidium sp. = 260, Cyclidium sp. = 158, E. aediculatus = 60, P. aurelia = 51, P. bursaria = 122, S. ambiguum = 0.4. We measured dispersal in 24 replicates of 2 bottles connected by silicone rubber tubing that was 13 cm long and 0.635 cm internal diameter. To complete the final count of dispersing individuals in a timely manner, we conducted 14 replicate dispersal trials on day 1 and 20 on day 2. Source bottles received 100 ml of the mixed species stock cultures. The recipient bottles were filled with 100 ml of sterile nutrient medium, rather than bacteria-rich nutrient medium, to encourage the organisms to move in search of food. Beginning 5 hours later (that is, before reproduction could happen for most Haddad et al., Supplementary Material, Page 2 species), we started randomly sampling the non-inoculated bottles. We homogenized and then surveyed the liquid until we counted at least eight individuals of each species. If 8 individuals were not found, as was the case for some of the poor dispersers, we surveyed all 100 ml of liquid in the bottle. We determined dispersal rank in the same way that Cadotte (2006) calculated rank from his measure of colonization, although our measure of dispersal was different. We first divided the number of individuals in the recipient bottle by the number in the source bottle to obtain a proportion that dispersed. We then ranked this proportion for each trial. Finally, we averaged the ranking among trials to determine dispersal rank for a species. When a species was tested in both days, we also averaged the dispersal rank across days. Dispersal ranks are reported in Table 1 of the main text. Dispersal rank was highly correlated with intrinsic growth rate (Table S1). Because this was a non-spatial experiment, we used intrinsic growth rate in analysis. Colonization rank Colonization ranks were available for 6 of our 8 species from Cadotte, et al. (2006). The ranks of Cadotte et al. (2006) were measured in a similar experimental setup to ours, with the response being the time to colonization of a more distant bottle. Like dispersal rank, colonization rank was correlated with intrinsic growth rate and dispersal rank. Furthermore, because we did not have colonization ranks for all species, we did not use colonization rank in analysis. Haddad et al., Supplementary Material, Page 3 Spreading speed and diffusion coefficient Because we were concerned that dispersal rank and colonization rank were not identical, and that dispersal rank was not significantly correlated with competitive rank, as Cadotte, et al. (2006) found with a similar group of protozoans (Table S1), we used the spreading speed as another metric of colonization using data we had for all of our species. The spreading speed is closely related to the measure of colonization used by Cadotte et al (2006). The spreading speed combines intrinsic growth rate and the diffusion coefficient into one metric and is equal to 2*(r*D)0.5 (e.g., Okubo & Levin 2002). Cadotte, et al. (2006) also recognized that colonization is related to dispersal and population growth. In our experiment, we assumed dispersal between two patches (i,j) was governed by dN i D N j N i dt yielding the diffusion coefficient: D 1 2 N 2 t (1) ln 1 2t N1 0 where t is the time of the trial, N1(0) is the starting density in the source bottle, and N2(t) is the final density in the recipient bottle. D and spreading speed were correlated with intrinsic growth rate, and because this was a non-spatial experiment, we used intrinsic growth rate in analysis. Competitive rank Independent of Cadotte, et al. (2006), we had conducted a similar competition experiment using pairwise comparisons of the subset of species used in this paper. Between Cadotte Haddad et al., Supplementary Material, Page 4 et al. (2006) and our experiment, we evaluated competitive rankings of all species used in analysis of species responses to disturbance in this paper. We first describe our competition experiment, and then how we scaled the two experiments to obtain one ranking. The outcome of competition among eight paired species was assayed in a fully crossed design. Species used were Rotifer sp., Paramecium aurelia, Coleps sp., Euglena gracilis, Paramecium caudatum, Euplotes aediculatus, Cyclidium sp., and Uronema sp. Treatments consisted of bottles containing standard nutrient media, as in the main experiment, with two wheat seeds to provide a slow release of nutrients. There were 4 replicates of each species pair and each single species for a total of 144 bottles. Approximately 30 individuals of each species were added to initiate the experiment. Densities of species were sampled after 4 weeks, sufficiently long for equilibrium densities to be reached, using the methods described for the main experiment. We defined competitive effect as the effect of an individual on another individual, whether of the same or a different species. We quantified competitive effect as the reduction in per capita growth rate caused by an individual. To calculate pairwise competitive effects, we used a two species Lotka-Volterra model, which at equilibrium is 1 dN iP* ri 1 ii N iP* ij N Pj* 0 P* Ni dt (2) where N iP* is the density of species i at equilibrium when grown in "pair" culture (i.e. with species j), and ij is the interspecific interaction coefficient (the per capita effect of Haddad et al., Supplementary Material, Page 5 species j on species i). We used this model because the population growth of our study microorganisms is best represented in continuous time. Solving for ij gives the following equation: ij 1 ii N iP* N iS* N iP* 1 P* N Pj* N iS* Nj (3) where ii is the intraspecific interaction coefficient and is equal to the reciprocal of the equilibrium density in single species culture, ii = 1/NiS*. Equation 3 shows that the interspecific competitive effect of species j is quantified as the per capita contribution of species j to the proportional reduction in the equilibrium density of species i in pair culture compared to single-species culture. When the sign of the interaction coefficient is negative, the effect is facilitative. For each replicate, we used the final densities to calculate ii for single species cultures and ij for all pairwise species combinations. We defined competitive ability, C, of a species as the combination of its average competitive effect in the community, and its average competitive response (ability to withstand competition), as follows Cj 1 ij ji n i i (4) where the first summation is the effect, the second summation is the response, and n is the number of species. Negative C indicates poor competitive ability, whereas positive C indicates good competitive ability. Zero C indicates that the net competitive ability of the species is neutral. Equation 4 shows that species with the poorest competitive ability have a facilitative effect on other species but also suffer from their competitive effects. Haddad et al., Supplementary Material, Page 6 Conversely, species with the best competitive ability strongly compete with other species but are also facilitated by them. Our measure of competitive ability is similar to Mouquet et al. (2004) and Cadotte et al. (2006) in that both effect and response contribute to overall competitive ability. Mouquet et al. (2004) used the relative yield of a single generational step. However, compared to their system of annual plants with a discrete time process, relative yield was not appropriate for our system in which competitive effect is continuous and incremental in time. Cadotte et al. (2006) calculated competitive ability from long term persistence data. For example, the effect of species j on i was considered to be high if i often went extinct in the presence of species j. Our measure more finely defines the competitive ability of each species by directly estimating per capita interaction coefficients, and has the advantage of shorter culture times. To obtain a ranking of the competitive ability of each species, we calculated Cj for one replicate set of single and pairwise cultures, and ranked the species from 1 to 8 from worst to best competitor. To estimate the mean and confidence limits for the rank of competitive ability, we used a bootstrap procedure. We first resampled the final density data to construct four new replicate data sets. In this resampling, individual trials for single or pairwise species could be drawn from any of the four original replicates. For each new replicate, we calculated one complete set of ijs, effects, responses, Cjs, and ranks. We then calculated the mean of the four replicates. We drew 10000 bootstrap sets of four replicates. We took the 2.5 and 97.5 percentiles from the bootstrap distribution of the means to determine the 95% confidence limits for the mean competitive rank. The bootstrap estimates are given in Tables S2 and S3. Haddad et al., Supplementary Material, Page 7 As in Cadotte, et al. (2006), competitive ranks were highly variable. However, ranks from the two studies were similarly ordered (Fig. S2). Thus, we rescaled the rankings from Cadotte, et al. (2006), using the 3 overlapping species among the two studies. Rescaling was necessary because ranks were based on different species numbers. Rescaling was accomplished by, first, conducting orthogonal regression to obtain the relationship: competitive rank = -11.85 + 2.15*(Cadotte et al. (2006) rank) and, second, averaging ranks of species that overlapped among the two studies. Unlike Cadotte, et al (2006), our measure of competitive rank was not significantly correlated with dispersal rank, so both variables could be safely included in analysis of effects of species traits on response to disturbance. We should note that we used fewer species in our experiment than Cadotte, et al. (2006), and with more species, given the trend we observed, there may have been a significant, negative relationship between competition and dispersal ranks. Correlations among variables Because of correlations between variables (Table S1), we used three variables in analysis, intrinsic growth rate, ln(K), and competitive rank. The intrinsic growth rate was related to every variable measuring dispersal or colonization. We used intrinsic growth rate in analysis because this was a non-spatial experiment. Ln(K) and cell size were also highly correlated, so we retained ln(K) because it had more ecological relevance in the context of this experiment. Finally, competitive rank was not correlated with any other variable we used in analysis. Pragmatically, having a reduced set of explanatory variables was Haddad et al., Supplementary Material, Page 8 reasonable given that our sample size in species trait analysis was 8 (one for each species). Regression analysis We used the three variables in multiple regression analysis. Each regression started with all three variables, intrinsic growth rate, ln(K), and competitive rank (weighted by the difference between the max and min of the 95%CI of competitive rank to account for variation in that variable). Variables were eliminated through backward elimination multiple regression if P>0.20. Results of regression analyses are presented in Tables S4S6. We note that there are interdependencies among how traits predict responses by disturbance type that are not accounted for in analysis. We did not conduct a full analysis with interactions because 1) our sample size is 8 so it is not possible to include many factors and interactions, and 2) intrinsic growth rate is the dominant predictor in all cases, which would not change in a more complicated analysis. Haddad et al., Supplementary Material, Page 9 Table S1. Correlations among traits. (Pearsons correlation coefficient) italics indicate p<0.05, bold typeface indicates p<0.01 Variable Ln(cell size) r Ln(r) Ln(K) Dispers al rank D Spreading speed Colonization rank Ln(cell size) -0.41 r Ln(r) Ln(K) Dispersal rank D Spreading speed Colonization rank (Cadotte) Competitive rank -0.46 0.97 -0.85 0.18 0.20 -0.50 0.95 0.93 0.30 -0.03 0.80 0.70 -0.09 0.83 -0.11 0.87 0.78 -0.05 0.89 0.99 -0.58 0.80 0.82 -0.10 0.80 0.78 0.77 0.24 -0.63 -0.58 -0.06 -0.61 -0.68 -0.67 -0.89 Table S2. Mean interaction coefficients (ij), mean competitive effects, and mean competitive responses of eight species estimated by bootstrap resampling. Table cells are the effect of species j (columns) on species i (rows). Negative values indicate facilitation. Rotifer sp. P. aurelia Coleps sp. E. gracilis P. caudatum E. aediculatus Uronema sp. Cyclidium sp. Comp effect Rotifer sp. 0.00187 -0.00146 0.0007 -0.04063 -0.00176 -0.0004 -0.00437 0.00159 -0.00556 Paramecium aurelia 0.00115 0.00333 0.00048 -0.01134 0.00678 0.00395 -0.00228 -0.00017 0.00024 Coleps sp. -0.11883 -1.62874 0.04324 0.01939 -0.01494 0.52281 -0.47764 0.00234 -0.20655 Euglena gracilis 8.6x10-7 0.00021 -0.02742 0.00067 0.00142 0.00016 -0.00088 -0.00041 -0.00328 Paramecium bursaria. 0.00256 0.02031 -0.00858 -0.00733 0.0052 -0.05365 -0.00526 -0.00119 -0.00599 Euplotes aediculatus -0.0027 0.23823 0.26251 0.02759 0.03059 0.02481 0.02038 0.18224 0.09795 Haddad et al., Supplementary Material, Page 10 Uronema sp. 0.00016 0.0005 -0.00032 -0.0004 0.00012 -0.06923 0.00137 -0.0003 -0.00851 Cyclidium sp. 0.00041 0.00016 0.00234 0.00032 -0.00004 0.03565 -0.00094 0.00081 0.00484 Comp. Resp. -0.01442 -0.17093 0.03412 -0.00147 0.00342 0.05801 -0.0587 0.02311 Table S3. Mean competitive ability and mean competitive rank of eight species estimated by bootstrap resampling. Negative values indicate poor competitive ability. Species Rotifer sp. Paramecium aurelia Coleps sp. Euglena gracilis Paramecium caudatum Euplotes aediculatus Uronema sp. Cyclidium sp. Competitive ability 0.00887 0.17117 -0.24066 -0.00182 -0.00876 0.03955 0.05019 -0.01827 Competitive rank 4.637 5.362 2.438 4.144 4.100 5.936 5.961 3.421 Table S4. Traits that predict species response to disturbance intensity. Ln(K) and competitive rank were eliminated in backward multiple regression. Source Model Error Corrected Total df 1 6 7 Sum of Squares 0.00004462 0.00002977 0.00007439 Parameter estimate -0.01179 0.004 Standard error 0.00146 0.00133 F 8.99 P 0.02 t -8.05 3.00 P 0.001 0.02 R2 = 0.60 Variable Intercept Intrinsic growth rate Table S5. Traits that predict species response to disturbance frequency. Competitive rank was eliminated in backward multiple regression. Source Model Error Corrected Total df 2 5 7 Sum of Squares 0.1536 0.07029 0.22389 Parameter estimate -0.00163 -0.18841 0.02967 Standard error 0.12764 0.06029 0.01775 F 5.46 P 0.055 t -0.01 -3.12 1.67 P 0.99 0.03 0.16 R2 = 0.69 Variable Intercept ln(r) ln(K) Haddad et al., Supplementary Material, Page 11 Table S6. Traits that predict species response to disturbance effects on nutrient loss. Ln(K) was eliminated in backward multiple regression. Source Model Error Corrected Total df 2 5 7 Sum of Squares 0.64116 0.04216 0.70662 Parameter estimate -0.21252 0.28153 0.02155 Standard error 0.05959 0.04216 0.007 F 24.49 P 0.003 t -3.57 6.68 3.08 P 0.02 0.001 0.03 R2 = 0.91 Variable Intercept Intrinsic growth rate Competitive rank Haddad et al., Supplementary Material, Page 12 Figure S1. Relationship between population size and growth rate for each species (A-H) studied in the disturbance experiment. Points show growth rates from 5 replicates each for high and low nutrient microcosms. Lines show the best fit estimates derived for r and K. Although we show all points from high and low nutrient treatments, r and K were determined separately for each microcosm and then averaged to generate the best fit line. Figure S2. Rescaled means and 95% confidence intervals of competitive ranks obtained from this and Cadotte et al’s (2006) studies. Filled symbols are for species shared among the two studies that were used to rescale ranks. Figure S3. Effects of disturbance intensity, frequency, and impacts on nutrient loss on the density of each species (A-H). Numbers within bars are the proportion of replicates (out of 10 total) in which the species persisted. Haddad et al., Supplementary Material, Page 13 0.8 Low nutrients High nutrients Predicted low Predicted high Log(Nt+1/Nt)/ (time interval)^0.5 0.6 0.4 0.2 B) Colpidium sp. 2 1 0 0 50 100 150 200 -0.2 0 0 -0.4 200 400 600 800 -1 -0.6 -2 -0.8 Log(Nt+1/Nt)/ (time interval)^0.5 3 3 C) Cyclidium sp. 2.5 2.5 2 2 1.5 1.5 1 1 0.5 0.5 0 D) Euglena gracilis 0 0 1000 2000 3000 4000 5000 -0.5 0 40000 80000 120000 160000 -0.5 0.6 E) Euplotes aediculatus F) Paramecium aurelia 1.5 Log(Nt+1/Nt)/ (time interval)^0.5 0.3 1.2 0 0 100 200 300 400 500 0.9 -0.3 0.6 -0.6 0.3 -0.9 0 0 -1.2 100 200 300 400 -0.3 0.65 G) Paramecium bursaria 1.4 H) Spirostomum ambiguum 0.45 Log(Nt+1/Nt)/ (time interval)^0.5 Figure S1 3 A) Coleps sp. 0.9 0.25 0.4 0.05 -0.15 0 150 300 450 600 750 900 -0.1 0 Haddad et al., Supplementary 14 Density Material, Page -0.35 -0.6 10 20 Density 30 40 Figure S2 Haddad et al. Euplotes P.aurelia Euglena Cyclidium Coleps Cadotte et al. (2006) P.bursaria P.aurelia Spirostomum Euplotes Coleps Colpidium -5 0 5 Mean rank Haddad et al., Supplementary Material, Page 15 10 Figure S3 1000 A) Coleps sp. Intensity=50% Intensity=98% Intensity=89% Density 100 High Nutrients Low Nutreints 10 1 0.1 0.01 1.0 Control 10000 0.9 1.0 0.9 Low 0.9 0.2 High 0.8 Low 0.0 1.0 High 0.2 0.0 0.1 Low 0.0 High B) Colpidium sp. Density 1000 100 10 1 0.1 0.01 1.0 Control 10000 1.0 0.9 1.0 Low 1.0 High 1.0 1.0 Low 1.0 0.8 0.9 High 0.6 Low 1.0 0.1 High C) Cyclidium sp. Density 1000 100 10 1 0.1 0.01 1.0 Control 10000 1.0 1.0 1.0 Low 1.0 High 1.0 0.9 0.9 Low 0.1 High 1.0 0.2 0.4 Low 0.0 High D) Euglena gracilis Density 1000 100 10 1 0.1 0.01 1.0 Control 0.9 1.0 0.8 Low 0.9 High Frequency 0.7 0.5 0.0 Low 0.1 High Frequency Haddad et al., Supplementary Material, Page 16 0.1 0.2 0.2 Low 0.0 High Frequency Figure S3 (cont.) 100 E) Euplotes aediculatus Intensity=89% Intensity=50% Intensity=98% Density 10 High Nutrients Low Nutrients 1 0.1 0.01 1.0 1.0 Control 1000 0.8 0.7 Low 0.9 0.8 High 0.9 0.0 Low 0.0 High 0.2 0.0 0.0 Low 0.0 High F) Paramecium aurelia Density 100 10 1 0.1 0.01 1.0 1.0 Control 1000 1.0 1.0 Low 1.0 1.0 1.0 High 1.0 Low 0.8 1.0 High 0.4 1.0 Low 0.0 High G) Paramecium bursaria Density 100 10 1 0.1 0.01 1.0 Control Density 10 1.0 1.0 1.0 Low 1.0 1.0 High 1.0 0.0 Low 0.0 1.0 High 0.8 0.0 Low 0.0 High H) Spirostomum ambiguum 1 0.1 0.01 0.8 Control 0.7 0.4 0.2 Low 0.1 High Frequency 0.1 0.0 0.0 Low 0.0 High Frequency Haddad et al., Supplementary Material, Page 17 0.0 0.0 0.0 Low 0.0 High Frequency Literature Cited Cadotte M.W., Mai D.V., Jantz S., Collins M.D., Keele M. & Drake J.A. (2006). On testing the competition-colonization trade-off in a multispecies assemblage. The American Naturalist, 168. Mouquet N., Leadley P., Meriguet J. & Loreau M. (2004). Immigration and local competition in herbaceous plant communities: a three-year seed-sowing experiment. Oikos, 104, 77-90. Okubo A. & Levin S.A. (2002). Diffusion and ecological problems: modern perspectives. Springer-Verlag, New York. Haddad et al., Supplementary Material, Page 18