Science Notes on Kinetic Theory
advertisement

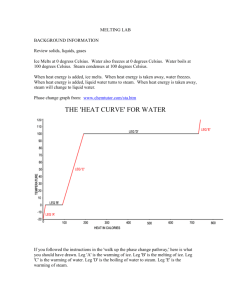
Science Notes on Kinetic Theory Kinetic Theory Theory – an explanation based on facts and observations. Theories are used to help us understand our world. They sometimes used models to assist understanding Kinetic Theory –states that all matter is made of atoms that are constantly moving - as energy is added, the atoms move faster - as energy is removed, the atoms move slower - if I keep adding energy, the atoms will continue to pick up speed, moving faster and faster - if I keep removing energy, the atoms will continue to slow down, moving slower and slower Temperature – temperature changes change density Hot objects are less dense than cold objects Temperature is the average speed of atoms in a substance Thermometer – used to measure temperature When a thermometer is put in contact with a surface, the atoms of the substance and the atoms of the thermometer begin colliding into each other; the atoms in the thermometer and substance begin to travel at the same speed Thermal expansion – how much an object expands or contracts when temperature changes; different materials will expand and contract in different amounts How does Energy get transformed? (Why does density change when temperature changes???) Energy always moves from hotter temps to lower temps When objects with 2 different temperatures are mixed, the faster atoms slow down and the slower atoms speed up. Where they touch, the energy gets transferred. History of the Thermometer One of the first known thermometers was invented by Galileo (1593) Galileo found that as things get cold, they contract and when the get warm/hot they expand Galileo put a glass bulb at the end of a long tube. When the bulb was heated or cooled, the liquid moved up and down the tube (Thermometer #1) Galileo had no numbers on his thermometer – was inconsistent, inaccurate and bulky How does Galileo’s thermometer work? As atoms gain energy, they go faster As they go faster the atoms collide into each other more vigorously As they begin to hit each other harder they begin to bounce further apart and the object expands NOTE Atoms do NOT change size. They move farther apart when heated and closer together when cooled. Different metals will expand and contract at different rates Galileo’s thermometers were not very accurate and they were bulky to use in experiments Later – Galileo improved his first attempt with a second thermometer based on density Galileo did not have the technology available later on Thermal Expansion – how much an object expands or contracts with temperature changes Different materials will expand and contract by different amounts Brass (high thermal expansion) will change a lot, but wood (has low thermal expansion and will not expand or contract much Example: Water (at room temperature) has a density of 1g/mL…BUT…when you heat it, the atoms move faster and farther apart. If I cool the water, the atoms move slower and closer together Density and Temperature Density is how tightly packed together atoms are If I heat something up, the atoms bounce around and spread out – that something becomes less dense When objects are colder, the atoms move slower and closer together Daniel Fahrenheit (1724) - First Accurate Thermometer/starts at 32 degrees F Fahrenheit decided to make a better thermometer than Galileo’s so he could sell them and make a profit Fahrenheit had 100 years more of technology behind his thermometer than Galileo Fahrenheit made his thermometer with the numbers on it and then began measuring the temperature of things People in Fahrenheit’s time knew how to make thinner glass and hotter fires He used a very small and skinny version of a test tube that was both portable and accurate He made a mixture of ammonium chloride, ice and salt (the coldest liquid he could come up with) and put a thermometer in it Stuck a thermometer in the mixture and marked the lowest point and called it “0” He then stuck that thermometer into ice and found that water freezes at 32 degrees Fahrenheit He then stuck the thermometer into boiling water and found that water boils at 212 degrees Fahrenheit Fahrenheit found 3 Fixed Points 1. Water freezes and melts at 32 degrees 2. Water boils at 212 degrees 3. Most people have a normal temperature of 98.6 degrees Next came – Anders Celsius – 1742 Anders didn’t like Fahrenheit’s thermometer…thought it was great…BUT… 1. It was difficult to reproduce 2. Math calculations were difficult 3. There was no pattern Wanted a metric thermometer based on water Celsius measured things to make his thermometer He took a blank thermometer and stuck it in freezing water He watched the mercury drop to the lowest point and marked it “0” – the point where water freezes He then stuck that thermometer into boiling water and watched the mercury rise to a point he labeled 100 degrees – and called it the boiling point So his thermometer had a bottom of 0 degrees (freezing) and a top of 100 degrees (boiling) It thus had 100 divisions and he called his thermometer a centigrade thermometer He based his thermometer on the metric system and a number system based on 10 – easy to make calculations It was called a centigrade or Celsius thermometer – both the same thing Today, this is the only thermometer used by scientists worldwide Body temperature on a Celsius thermometer is 37 degrees (Mercury is more sensitive to temperature so it makes the best thermometer, but is not used much anymore because it is toxic – can be absorbed through the skin or breathed in Next is Lord Kelvin – 1848 (William Thomson – knighted Lord Kelvin by the queen) Used Celsius’ thermometer, but started at the coldest temperature possible…..Absolute Zero ABSOLUTE ZERO =0 DEGREES KELVIN (which in Celsius degrees would be -273) Starting at Absolute zero, he made his freezing point 273 degrees Kelvin and his boiling point 373 degrees Kelvin Found that as temperature decreases (and things get colder) pressure goes down Found that as temperature increases (and things get hotter) pressure goes up Found that removing heat (energy) would result in the atoms slowing down Asked if you keep removing energy and the atoms keep slowing down, at what temperature would the atoms stop moving? This would be ABSOLUTE ZERO – 0 DEGREES KELVIN We have never reached absolute zero but did come close September of 2003, scientists at MIT chilled a cloud of sodium atoms to a record-breaking 0.45 nanokelvin – that is .00000000045 K RE-CAP 4 thermometers beginning with … 1. Galileo’s thermometer –a glass bulb at the end of a long tube…bulky and not very accurate 2. Fahrenheit – first accurate thermometer; based on the properties of water; has many numbers on it and goes from 0 degrees to well over 100 degrees; water freezes at 32 degrees and boils at 212 degrees on the Fahrenheit thermometer 3. Celsius – based on water also; begins at 0 degrees (the freezing point of water) and ends at 100 degrees (the boiling point of water) 4. Kelvin – also based on the properties of water; starts at absolute zero and goes from 273 degrees (freezing point of water) to 373 degrees (boiling point of water) CONVERTING CELSIUS TO KELVIN and KELVIN TO CELSIUS K = C + 273 C = K -273 PLANK TEMPERATURE – just as Kelvin theorized about absolute zero (the coldest temperature possible), there is also a theory of the hottest temperature possible and it is called Plank Temperature This temperature is 1.4 x 10 to the 32nd power K AT THIS TEMERATURE ALL MATTER IS DESTROYED INTO A MIXED MUSH OF INDISTINGUISHABEL HOT ATOMIC SLUDGE. ATOMS WOULD BE MOVING SO FAST THAT THEY WOULD LOSE THEIR IDENTITY SCIENTISTS HAVE CREATED THE HOTTEST TEMPERATURE EVER RECORDED INSIDE A REACTOR AT 4 TRILLION DEGREES – 250,000 TIMES HOTTER THAT THE CORE OF OUR SUN. THREE FIXED POINTS IN FARENHEIT – CELSIUS – KELVIN F C K BODY TEMPERATURE 98 37 310K WATER FREEZING 32 0 -273K WATER BOILING 212 100 373K


![Temperature Notes [9/22/2015]](http://s3.studylib.net/store/data/006907012_1-3fc2d93efdacd086a05519765259a482-300x300.png)