Problem - King Fahd University of Petroleum and Minerals
advertisement

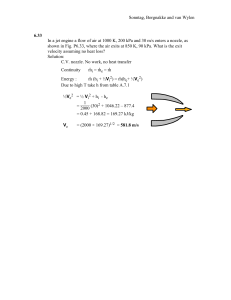
King Fahd University of Petroleum and Minerals Mechanical Engineering Department Thermodynamics II (ME204) Final Exam (Term 011) 13-10-2001 9th Jan., 2002 Duration: 2 hours 30 minutes Student Name: ID # : Serial # : Dr. M. A. Antar Closed Book/Closed Notes Exam A Formula sheet is provided Problem Mark 1 2 3 4 Total grade 25 25 25 25 100 This exam contains 9 pages including the cover page. Note, for SSSF process v2 v2 Q m i hi i gz i W m e he e gz e 2 2 For Ideal Gas T P s 2 s1 c P ln 2 R ln 2 T1 P1 Grade Earned Problem # 1 In a particular reheat-cycle power plant, steam enters the high-pressure turbine at 5 MPa, 450C and expands to 0.5 MPa, after which it is reheated to 450C. The steam is then expanded through the low-pressure turbine to 7.5 kPa. Liquid water leaves the condenser at 30C, is pumped to 5 MPa, and then returned to the steam generator. Each turbine is adiabatic with an isentropic efficiency of 87% and the pump efficiency is 82%. If the total power output of the turbines is 10 MW. a. Sketch the T-s diagram & Determine the mass flow rate of steam b. Determine the pump power input c. Determine the thermal efficiency of the power plant. d. If the ambient condition is 25 oC and 0.1 MPa, determine the availability of the turbine. Problem # 2 A flow moist air at 100 kPa, 40C, 40% relative humidity is cooled to 15C (where 2 = 100 %) in a constant pressure SSSF device. a. Find the humidity ratio of the inlet flow. b. Find the humidity ratio of the exit flow. c. Find the heat transfer in the device per kg dry air. Problem # 3 Develop an expression for the variation in temperature with pressure in a constant entropy process, ( T/ P)s , that only includes the properties P–v–T and the specific heat, Cp . An uninsulated compressor delivers ethylene, C2 H4 , to a pipe, D 10 cm, at 10.24 MPa, 94C and velocity 30 m/s. The ethylene enters the compressor at 6.4 MPa, 20.5C and the work input required is 300 kJ/kg. Find a. Ze and specific volume ve. b. The mass flow rate, c. The total heat transfer d. Entropy generation, assuming the surroundings are at 25C. Additional sheet for Problem # 3 Problem # 4 In a new high-efficiency furnace, natural gas, assumed to be 90% methane and 10% ethane (by volume) each enter at 25C, 100 kPa, and the products (assumed to be 100% gaseous) exit the furnace at 77C, 100 kPa. a. Write the Stoichiometric combustion equation. b. Write the combustion equation for 110 % theoretical air. c. Consider 110 % theoretical air case, what is the heat transfer for this process? d. Compare the heat transfer rate to that of an older furnace where the products exit at 227oC, 100 kPa. Formula Sheet Chapter 10: rev Q0 = T o ( S2 S1 )1 Q2 Reversible heat transfer for a control mass: T0 TH W 2 = 1 Q2 Q0rev (U 2 U 1 ) T o ( S 2 S1 ) (U 2 U 1 ) 1 Q2 (1 rev Reversible work for a control mass: 1 Irreversibility for a control mass: 1 I 2 = 1W 2rev 1W 2ac = T o ( S 2 S1 ) dS c.v. Reversible heat transfer for an SSSF process: Q c.v.,0 = T o dt rev T0 1 Q2 ] T0 S net 1 Q 2 T0 [( S 2 S 1 ) TH TH T + m e s e - m i s i - o Q c.v, H TH i htot,i - T 0 si - m e htot,e - T 0 s e + 1 Reversible work for an SSSF process: W c.v.= m rev where htot h + T 0 Q c.v.H TH 1 2 V + gZ 2 ac rev ac Irreversibility for an SSSF process: Ic.v.= W c.v. - W c.v.= Q - Qc.v.,0 T 0 S gen,c.v. c.v.,0 rev 1 2 V + gZ - ho - T 0 s0 + gZ 0 2 T 0 ac Ic.v.= m i i - m e e + 1 Q c.v.,H - W c.v. TH Flow availability: = h - T 0 s + Non-flow availability: = e - T 0 s - eo - T o so + Po v - vo e + P0 v - T o s - e0 + P0 v0 - T 0 s0 e u + V2/2 + gZ where T o ac I 2 = m 1 - 2 + 1 .1 Q 2,c.v.,H - .1 W 2 - P0 ( V 2 - V 1 ) TH 1 Second-law efficiency: 2nd wa for turbine; law = i -e P-v relations for ideal gas (with constant Cp): 2nd law = and n 1 1 Chapter 12: Mass fraction: ci = Mole fraction: yi = y Mi /n mi n n = i M i = i M i tot = i mtot ni M j ni M j / ntot y j M j ni mi / M i mi / ( M i mtot ) c / = = = i Mi ntot m j / M j m j / ( M j mtot ) c j / M j Relative humidity: = Pv = v = v g P g g vv Humidity ratio: = 0.622 = Pv Pa Pa 0.622 P g m A A n 2 for heat exchangers. m B B T 2 p2 Pv = constant = P v = P2 v = T 1 p1 n T0 ) TH n 1 / n v = 1 v2 n -1 Chapter 13: x y = 1 y z x z Mathematical relations: T P = - , v s s v Maxwell relations: x y z = - 1 y z z x x y and T v = , P s s p s g - s f s fg h fg dP = = = dT sat v g - v f v fg Tv fg Clapeyron equation: Reduced temperature, pressure and volume: P r = P s = , T v v T P Pc , Tr= ln T Tc P2 h fg (T2 T1 ) P1 R (T1 T2 ) , vr = v v dh C p dT v T ( ) P dP T The change in internal energy: P du C v dT T ( ) v P dv T The change in entropy: ds C v dT P ( ) v dv T T and * * * * h1 - h2 = - h1 - h1+ h1 - h2 + h2 - h2 * * * * s1 - s 2 = - s1 - s1+ s1 - s 2 + s 2 - s 2 Chapter 14: Fuel-air ratio: AF mass = mair m fuel and AF mole = nair n fuel Equivalence ratio: = FA / FAs = AFs / AF h RP = H P - H R Enthalpy of combustion: o o h RP = ne h f + h e - ni h f + h i P R P.v = ZRT vc The change in enthalpy: Equations for generalized tables: v s = - . T P P T ds C P dT v ( ) P dP T T