Molar Volume of a Gas
advertisement

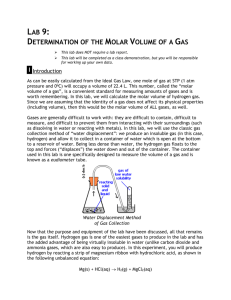
Molar Volume of a Gas Pre-Lab Discussion: Avogadro’s hypothesis states that equal volumes of all gases contain equal numbers of molecules under the same conditions of temperature and pressure. It follows from this hypothesis that all gas samples containing the same number of molecules will occupy the same volume under the same conditions of temperature and pressure. A special name is given to the volume occupied by 1-mole samples of gases at STP (standard temperature and pressure). This volume is called the molar volume. In this experiment, you will make an experimental determination of the molar volume. The basis of this experiment is the following unbalanced reaction, in which you will react a known mass of magnesium with excess hydrochloric acid to produce the substances shown: Mg(s) + HCl (aq) MgCl2 (aq) + H2 (g) The hydrogen gas is the product that is of interest to you in this\experiment. You will make an experimental determination of the number of moles of hydrogen molecules produced and the volume occupied by these molecules. The number of moles of hydrogen will be determined indirectly. The balanced equation for this reaction shows the mole ratio of magnesium reacted to hydrogen gas. (Think stoichiometry). Therefore, by determining the mass of magnesium that reacts and the number of moles that this mass is equal to, you will also determine the number of moles of hydrogen gas produced. The volume of hydrogen gas produced will be measured directly on the scale of a gasmeasuring tube. (The eudiometer). The gas laws of Boyle and Charles will be used to correct this volume, measured under laboratory conditions, to the volume the sample of gas would occupy at STP. The collected data (number of moles and volume at STP) will be used to calculate the molar volume of the hydrogen gas. This experiment should aid in the understanding of the mole concept and the concept of molar volume of a gas. Pre-Lab: Complete the following in your lab notebook: 1) Title of Lab (Descriptive!!) 2) Objective of Lab (Brief!!) 3) Balanced Equation of chemical reaction 4) Detailed procedures to ensure another person could repeat the lab accurately. Equipment • Eudiometer (gas collection tube) • one-hole stopper (to fit tube) • ring stand • utility clamp • thermometer • beaker, 400 ml • large beaker • graduated cylinder, 10ml • metric ruler • safety goggles • 3M HCl • Magnesium ribbon Safety: Handle the 3M HCl with care; always wear your safety goggles when working with strong and/or concentrated acids. Note the caution under the procedures and follow the procedures carefully. Handle the gas-measuring tube with care! You break it = You buy it! Procedures: 1. Obtain a piece of magnesium ribbon no longer than 2.5 cm. Using the Analytical balance (the “fancy one”), obtain the mass (grams) of your piece of magnesium ribbon. 2. Obtain a piece of thread 15 cm long. Tie the end of the thread or wire around the piece of the Mg ribbon, leaving about 10 cm of thread/wire free. Bend the piece of the magnesium so that it will fit easily into the eudiometer. 3. Obtain about 10 ml of 3M HCl. CAUTION: Handle this acid with care!! Carefully pour the HCl into the gas-measuring tube. 4. Tilt the Eudiometer slightly. Using a beaker, slowly fill (yes, to the tippy-top) the eudiometer with water at room temperature. Pour slowly so as to avoid mixing the acid and water as much as possible. 5. Measure the temperature of the water in your eudiometer to the nearest .2°C – Record this temperature as “room temperature”. You’ll need this to determine the water vapor. 5. Lower the piece of Mg ribbon 4 or 5 cm into the eudiometer. Drape the thread over the edge of the tube and insert the one-hole rubber stopper into the tube as shown in Figure 1. 6. Add about 300 ml of water at room temperature to a 400 ml beaker. Set up a ring stand and utility clamp, and place the beaker of water in the position shown in figure 2. 7. Place your finger over the hole in the rubber stopper and invert (turn upside down) the eudiometer. Lower the stoppered end of the tube into the beaker of water. Clamp the tube in place so that the stoppered end is a few centimeters above the bottom of the beaker. Record your visual observations in your data table. 8. Let the apparatus stand about 5 minutes after the magnesium has completely reacted. Then, tap the sides of the eudiometer to dislodge any gas bubbles that may have become attached to the sides of the tube. 9. On the scale of the eudiometer, read the volume of the gases in the tube. Record this volume in your data table. 10. In your data table, record the temperature of the water and the barometric pressure. Molar Volume of a Gas Post Lab Assignment Use the data you obtained from your experiment to answer the following questions. Be sure to show all work & use appropriate units and significant figures. Calculations: 1. Find the mass of the Mg strip: 2. Calculate the number of moles of Mg reacted: 3. Find the pressure exerted by the H2 gas in the tube: i. PH2O is determined from the “Chem Bible” PH2 = PBarometric - PH 2O 4. Convert room temperature from °C to Kelvin: 5. Find the volume of the H2 gas at STP: P1V1 PSTPV = T1 TSTP 6. Find the volume of 1 mole of H2 gas at STP: **From your experimental data!! VH 2 ( from # 5) nH 2 ( from # 2) = VUnknown 1mole Conclusions and Questions: 1. The accepted value for the molar volume of a gas is 22.4 liters. How does your experimentally determined value compare with this accepted value? Calculate your percent error. 2. 3. What are some possible sources of error in this experiment? Find the volume of the following masses of gases at STP: **Show all work using FLM** a) 50.0 g O2 b) 15 g H2 c) 28.0 g N2 d) 75 g CO2 4. How many liters would the following number of moles of any gas occupy at STP? **Show all work using FLM a) 0.75 mole b) 0.85 mole c) 1.3 mole d) 2.7 moles e) 3.4 moles