SCH4U1_06_04b_Redox lab short
advertisement

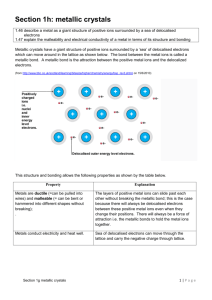
SCH4U1 Name: ________________ OXIDATION-REDUCTION REACTIONS PURPOSE: The purpose of this lab is to study the results of several oxidation-reduction reactions and rank the reactants based on of their relative strength as oxidizing or reducing agents. BACKGROUND: Zinc metal is oxidized to Zn2+ ions when it is placed in a solution of Ag+ ions. In the process, Ag+ ions are reduced to silver metal. Thus, in this reaction, there is a transfer of electrons from Zn(s) to Ag+ (aq). The Zn(s) is the reducing agent because it reduced the Ag+ (aq) ion. The Ag+ (aq) ion is the oxidizing agent because it oxidized the Zn(s). oxidation reduction + 2Ag (aq) net reaction 2Ag+ (aq) Zn2+ (aq) 2Ag(s) + 2e− + Zn (s) 2e− + Zn (s) 2Ag (s) + Zn2+ (aq) Part 1: In Part 1 this experiment, you will analyze the results of some possible oxidation-reduction reactions involving several metals and metallic ions. Various metals (copper, lead and zinc) were placed in solutions of copper (II) nitrate, lead (II) nitrate or zinc nitrate. The results of these tests are shown in Table 1 below. You will determine which metallic ions can remove electrons from which metals. From this information, you will be able to arrange the metallic ion-metal half-reactions in order of decreasing ease of reduction. Part 2: In Part 2, reactions were carried out between several halogen elements (chlorine, bromine and iodine) and the salts sodium chloride, potassium bromine and potassium iodide. In the experiment, the non-polar halogen product was extracted using the non-polar solvent cyclohexane and the colour of the organic layer was used to determine if a reaction occurred. The results will be used to make a similar comparison of the relative ease of reduction of the three halogen elements C12, Br2 and I2. Specifically, you will determine which of these halogens is capable of removing electrons from which of the halide ions, Cl− (aq), Br− (aq) or I− (aq). From this information, you will be able to arrange the halogen element-halide ion half-reactions in order of decreasing ease of reduction. OBSERVATIONS: Part 1- Reduction of Metaliic lons SOLUTIONS COPPER (Cu (s)) Cu(NO3)2 (aq) Pb(NO3)2 (aq) No reaction. Zn(NO3)2 (aq) No reaction. METALS LEAD (Pb (s)) Copper metal forms. Lead strip corrodes. ZINC (Zn (s)) Copper metal forms. Zinc strip corrodes. Lead metal forms. Zinc strip corrodes. No reaction. Part 2 - Reduction of Halogens SALT SOLUTIONS: Cl2 (aq) NaCl (aq) KBr (aq) Brown Br2 layer forms. KI (aq) Purple I2 layer forms. HALOGEN SOUTIONS Br2 (aq) I2 (aq) No reaction No reaction No reaction. Purple I2 layer forms. ANALYSIS: Part 1: 1) For each of the reactions that occurred in Part 1, write a balanced chemical equation. 2) For each reaction that occurred, write the net ionic equation and indicate the direction of electron movement using an arrow. 3) Which of the metallic ions was reduced by: a) two of the metals b) one of the metals c) none of the metals? 4) For each net ionic equation from question 2,, write the oxidation and reduction half reactions. 5) Arrange the metallic ion-metal reduction half-reactions in a column in order of decreasing ease of reduction (i.e. the metallic ion that was always reduced is at the top). Other experiments show that the Ag+ (aq) ion is reduced by Zn (s), Pb (s) and Cu(s). Use this information to add the half-reaction Ag+ (aq) + e− → Ag(s) to your list in the proper place. Part 2: 1) For each of the reactions that occurred in Part 2, write a balanced chemical equation. 2) For each reaction that occurred, write the net ionic equation and indicate the direction of electron movement using an arrow. 3) Which of the halogens tested was reduced by a) two of the halide ions b) one of the halide ions c) none of the halide ions? 4) For each net ionic equation from question 2, write the oxidation and reduction half reactions. 5) Arrange the halogen element-halide ion half-reactions in a column in order of decreasing ease of reduction (i.e. the halogen that was always reduced is at the top). CONCLUSION: Construct a series of all seven half-reactions discussed in this experiment. List them in order of decreasing ease of reduction, given the following information: Ag+ (aq) ions are less easily reduced than Br2(aq) and more easily reduced than I2(aq); also I2(aq) is less easily reduced than Ag+(aq) and more easily reduced than Cu2+ (aq). QUESTIONS: Use the list you constructed in the conclusion to answer the following: a) Would it be possible to store a solution of copper(II) sulfate in a container made of metallic zinc ? Explain why or why not. b) Would it be possible to store a solution of copper(II) sulfate in a container made of metallic silver? Explain. c) Would you expect jewelry made from an alloy of silver and copper to tarnish (oxidize) in a laboratory where fumes of bromine are present? Explain your reasoning.