32. Determination of Electrochemical Series
advertisement

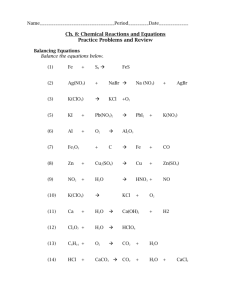
Name Period Date Lab: Determination of Electrochemical Series* Driving Question How do batteries make electricity? Materials and Equipment For each student or group: Data collection system Circular filter paper, 11- cm diameter Voltage sensor 1.0 M Zinc sulfate (ZnSO4), 10 mL Beaker (6), 50-mL 1.0 M Iron sulfate (FeSO4), 10 mL Glass plate (5 5 in) 1.0 M Copper sulfate (CuSO4), 10 mL Disposable droppers (6), 1 mL 1.0 M Silver nitrate (AgNO3),10 mL Iron strip, 1-cm 1-cm 1.0 M Lead nitrate (Pb(NO3)2), 10 mL Lead strip, 1-cm 1-cm 1.0 M Sodium nitrate (NaNO3), 20.0 mL Copper strip, 1-cm 1-cm Sand paper Silver wire, 1-cm Scissors Zinc strip, 1-cm 1-cm Safety Add these important safety precautions to your normal laboratory procedures: Dispose of solutions properly. Wear safety goggles throughout this activity. In case of contact with skin, chemicals should be washed off with large amounts of water. * This is an AP Chemistry course recommended experiment. 1 Determination of Electrochemical Series Procedure Set Up 1. Draw five small circles with connecting lines on a piece of circular filter paper (11-cm diameter), as shown in Figure 1. Label the circles M1, M2, M3, M4, and M5. M1 M5 M2 M4 M3 Figure 1: Filter paper diagram 2. Using a pair of scissors, cut wedges between the circles as shown. 3. Place the filter paper on top of the glass plate. 4. Obtain pieces of each of the five test metals. Sand each piece of metal on both sides so that a good electrical connection can be made. 5. Using a separate dropper for each solution, place three drops of each metal ion solution on the appropriate circle (M1, M2 etc.). Then, according to Table 2, place the corresponding piece of metal on the spot with its respective cation. The top side of the metal should be kept dry. These are the electrodes. Table 2: Metals and salt solutions for setting up each half-cell Material M1 M2 M3 M4 M5 Metal Copper Zinc Lead Silver Iron Zinc sulfate Lead nitrate Silver nitrate Iron sulfate Salt Solution Copper sulfate 6. Add enough 1.0 M sodium nitrate (NaNO3) solution to make a continuous trail along a line drawn between each circle and the center of the filter paper. You may have to dampen the filter paper with more NaNO 3 during the experiment. Note: The NaNO3 trace is the liquid-to-liquid junction between the electrodes. Any two of the electrodes coupled represent a galvanic cell. 2 PS-2897B Student Inquiry Worksheet Collect Data Use M1 (copper) as the reference metal. You will measure the potential of four cells by connecting M 1 to M2 (copper to zinc), M1 to M3 (copper to lead), M1 to M4 (copper to silver), and M1 to M5 (copper to iron). 9. Start a new experiment on the data collection system by clicking on “Sparkvue”. 10. Connect a voltage sensor to the data collection system. 11. Click on : “Build”. Then click: “Voltage” and arrow on the bottom and finally “OK”. On the next screen click on the green to begin recording voltage. 12. Touch the tip of the red (+) wire of the voltage sensor to one metal sample (for example, M1) and the tip of the black (–) wire to the other metal sample (for example, M2). If the voltage reading is below 0.00 V, reverse the ends of the voltage sensor, that is, switch the red (+) end of the sensor to M 2 and the black (–) end of the sensor to M1. 13. When the voltage reading stabilizes, record the voltage for the half-cell (half-reaction) combination and the color of the lead, or clip that is touching each of the metals, in Table 4 in the Data Analysis section. 14. Use the same procedure to measure the potential of the other three "half cells" with copper, M1, as the reference electrode. Note: If you get a voltage reading of 0.00 V or a fluctuating reading, add more NaNO3 solution along the lines connecting the metal spots. 15. Analyze your data for copper and make predictions about the other possible half-cell combinations using the same metals and solutions you used in this experiment. Data Make a table in your lab notebook where you can record the metal that is the cathode (red wire), the metal that is the anode (black wire) and the measured voltage. Data Analysis Your conclusion should include the following: A comparison of the measured values to the expected values. Explain the purpose of the sodium nitrate. A ranking of the metals from easiest to hardest to oxidize. 3