Notes 8-1 - RHS Academic Chemistry
advertisement

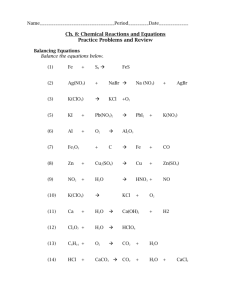
Name________KEY______________ Period_____ Notes 8-1: Introduction to Chemical Reactions Video Notes: 1. A new substance is formed in a chemical reaction. 2. List the signs of a chemical change. Color change, precipitate form, heat/light produced, gas produced 3. Identify the reactants and product in the following equation: C2H4 + 3O2 2CO2 + 2H2O Reactants Products 4. The Conservation of Mass states that mass cannot be created or destroyed in a chemical reaction. 5. What does a coefficient indicate? Give an example. The number of atoms/molecules/formula units in a chemical equation. An example would include 2 H2O means that there are two water molecules. 6. Briefly describe four major chemical reactions. Synthesis – 2 substances combine to form a new substance Decomposition – a substance breaks down into 2 or more new substances Single Replacement – atoms of one element replaces atoms of another element in a compound Double Replacement – atoms of 2 different compounds trade places with each other 7. Energy cannot be created or absorbed. In chemical reactions, energy changes forms. Endothermic reactions absorb energy, and exothermic reactions release energy. 8. Define reaction rate. The speed at which reactants turn into products. 9. List four factors that affect the rate of a reaction. Temperature (increase, increase), surface area (increase, increase), concentration (increase, increase), catalysts (increase) Writing Chemical Equations Step 1: Write the word equation (includes names and symbols) Step 2: Write the formula (skeleton) equation (includes formulas and symbols – unbalanced) Use nomenclature rules for compounds. Elements (pure substances consisting of only one type of atom) o Most elements are written using their symbol. Examples: o Exceptions Diatomic molecules (MEMORIZE!) Sulfur is _____ since that is how it is normally found in nature. Phosphorus is ____ since that is how it is normally found in nature. Step 3: Balance the reaction using ______________ (we’ll do this over the next few days!) Do NOT change any of the formulas! Examples: 1) A solution of potassium chromate is combined with a solution of silver nitrate. The precipitate silver chromate is produced as well as an aqueous solution of potassium nitrate. Word equation: Formula (skeleton) equation: 2) Solid copper metal reacts with aqueous silver nitrate to produce solid silver metal and aqueous copper II nitrate. Word equation: Formula (skeleton) equation: 3) Solid zinc metal reacts with aqueous copper II sulfate to produce solid copper metal and aqueous zinc sulfate. Word equation: Formula (skeleton) equation: More Examples: Translate the following equations into sentences. 1) 2 ZnO (s) + C (s) 2 Zn (s) + CO2 (g) 2) Na2O (s) + 2 CO2 (g) + H2O (l) 2 NaHCO3 (s) Name__________________________ Period_____ Notes 8-1: Introduction to Chemical Reactions Video Notes: 1. A __________ substance is formed in a chemical reaction. 2. List the signs of a chemical change. 3. Identify the reactants and product in the following equation: C2H4 + 3O2 2CO2 + 2H2O 4. The Conservation of Mass states that mass cannot be ___________ or ___________ in a chemical reaction. 5. What does a coefficient indicate? Give an example. 6. Briefly describe four major chemical reactions. Synthesis – Decomposition Single Replacement – Double Replacement – 7. Energy cannot be created or absorbed. In chemical reactions, energy changes forms. Endothermic reactions ___________ energy, and exothermic reactions _________ energy. 8. Define reaction rate. 9. List four factors that affect the rate of a reaction. Writing Chemical Equations Step 1: Write the word equation (includes names and symbols) Step 2: Write the formula (skeleton) equation (includes formulas and symbols – unbalanced) Use nomenclature rules for compounds. Elements (pure substances consisting of only one type of atom) o Most elements are written using their symbol. Examples: o Exceptions Diatomic molecules (MEMORIZE!) Sulfur is _____ since that is how it is normally found in nature. Phosphorus is ____ since that is how it is normally found in nature. Step 3: Balance the reaction using ______________ (we’ll do this over the next few days!) Do NOT change any of the formulas! Examples: 4) A solution of potassium chromate is combined with a solution of silver nitrate. The precipitate silver chromate is produced as well as an aqueous solution of potassium nitrate. Word equation: Formula (skeleton) equation: 5) Solid copper metal reacts with aqueous silver nitrate to produce solid silver metal and aqueous copper II nitrate. Word equation: Formula (skeleton) equation: 6) Solid zinc metal reacts with aqueous copper II sulfate to produce solid copper metal and aqueous zinc sulfate. Word equation: Formula (skeleton) equation: More Examples: Translate the following equations into sentences. 3) 2 ZnO (s) + C (s) 2 Zn (s) + CO2 (g) 4) Na2O (s) + 2 CO2 (g) + H2O (l) 2 NaHCO3 (s)