Supplementary Information (doc 1053K)
advertisement

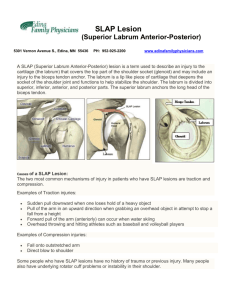
On-line Supplement Figure S1 Lack of Effect of Membrane-targeted Forms of c-Cbl on v-Abl-transformed Fibroblasts. (A) Cell lysates from v-Abl-3T3 cells transduced to stably express wild-type c-Cbl or membranetargeted c-Cbl-CAAX (both HA-tagged) were analyzed by Western blotting (WB) with antibodies as indicated. EF-1 was used as loading control. Individual cell clones used in this experiment are indicated at the top of the panel. (B) Cytosolic and membrane fractions were obtained from v-Abl-3T3 cells shown in (A) as described in the legend to Figure 1 and analyzed by WB with anti-HA. (C) v-Abl-3T3 cells ectopically expressing either wild-type c-Cbl or c-CblCAAX or N-Myr c-Cbl were plated on FN, and their morphology was analyzed as described in the legend to Figure 5C. Designations of individual clones are shown by the corresponding images. A representative experiment of two independent experiments is shown for each panel. Materials and Methods Cells v-Abl-transformed NIH3T3 fibroblasts stably expressing c-Cbl and Tyr5->Phe c-Cbl were generated by retroviral transduction and have been described previously (Feshchenko et al., 1999). Jurkat human T leukemic cell line, and COS-7 embryonic kidney cell line from the African green monkey were obtained from ATCC. Jurkat cells were grown in RPMI1640 supplemented with 10% fetal bovine serum. v-Abl-3T3 cells were grown in DMEM with 10% calf serum and 1g/ml puromycin (SIGMA, St. Louis, MO). COS-7 cells were grown in DMEM supplemented with 10% fetal bovine serum. The growth medium for the cells also contained 2mM L-glutamine, 100IU/ml penicillin, 100g/ml streptomycin and 20mM HEPES buffer (Mediatech, Herndon, VA). COS-7 cells were transfected with DNA constructs using DMRIE-C reagent (Invitrogen/Life Technologies, Carlsbad, CA). The efficiency of transfection was determined as the percentage of green fluorescent cells following co-transfection of pEGFP-C2, a mammalian expression plasmid encoding for enhanced green fluorescent protein (Clontech, Palo Alto, CA); it remained stable throughout the reported experiments at a level of 70-80%. COS-7 and v-Abl-3T3 cells were activated, where indicated, with pervanadate as described previously (Feshchenko et al., 1998). Transfection of v-Abl-3T3 cells with pre-designed SLAP specific siRNA (Ambion, Austin, TX) targeting different exons and two non-specific control siRNA, one labeled with Cy3 to monitor transfection efficiency, was done using X-treme transfection reagent (Roche Applied Science, Indianapolis, IN) following manufacturer’s recommendations. DNA constructs and mutagenesis SLAP was cloned from Jurkat T cells. Total RNA was isolated using RNAqueous-4PCR kit (Ambion), and the first strand of cDNA was synthesized using oligo dT primers and AMV reverse transcriptase (Promega, Madison, WI). This was followed by amplification using Advantage-HF2 PCR kit (Clontech) with nested primers: 5’GGG AAA AAG AAA GAA ATG GG 3’ (sense) and 5’TGT CTG TTC TTG GCT AGT C 3’ (anti-sense), 5’ GGA CGC GTC GAC ATG GGA AAC AGC ATG AAA TCC ACC CCT 3’ (sense) and 5’ TCC CCC GGG CTA TTT ATC ATC ATC ATC TTT GTA ATC TCT AGA TCT TGG CGA GTC CTC AAA GTA AGG 3’ (anti-sense) to obtain SLAP cDNA tagged with FLAG epitope in the C-terminus. The PCR product was cloned into pAlter-MAX vector (Invitrogen/Life Technologies). Positive clones were selected and sequenced on both strands. Mutations were introduced into SLAP using Pfu Turbo DNA polymerase (Stratagene, LaJolla, CA). The deletion of C-terminus of SLAP (amino acids 214-276) was performed as described by (Wang & Wilkinson, 2001) using primers 5’Phos-CCA AGA TCT AGA GAT TAC AAA GAT GAT GAT GAT AAA TAG CCC GG 3’(sense) and 5’Phos-TCC CTC GGG GTC CTC CTG CAG TCT GGA CAC TCT CCT 3’ (anti-sense). The G2A mutant was obtained using the single primer method (Marakova et al., 2000). The primer for G2A substitution was 5’ ACG CGT GGT ACC TCA AGA GTC GAC ATG GCA AAC AGC ATG AAA TC 3’. The primer was designed to additionally introduce a silent mutation 14 bp upstream of the start codon (TCT>TCA) to allow for screening of mutants. All mutations were verified by sequencing on both strands of DNA products. All SLAP coding sequences are FLAG-tagged. For generation of vectors for stable expression, SLAP and its mutants were recloned into pCEIII, a lentiviral gene transfer vector. The obtained vectors were delivered to v-Abl-3T3 cells expressing ectopic c-Cbl (Clone A4) using the previously described system (Hasham & Tsygankov, 2004). The transduced cells were sorted on the basis of EGFP expression, and SLAP expression was subsequently confirmed in the sorted cells by Western blotting. Expression plasmids for c-Cbl have been described previously (Feshchenko et al., 1999; Teckchandani et al., 2001). Briefly, Tyr5->Phe c-Cbl is full-length c-Cbl in which tyrosines 674, 700, 731, 735 and 774 are mutated to phenylalanines. HUT-Cbl, 480-Cbl, v-Cbl and (358-906)Cbl are truncation mutants encompassing c-Cbl amino-acid residues 1-655, 1-480, 1-357 and 358-906, respectively. G306E v-Cbl is the v-Cbl mutant, which harbors a point mutation that inactivates the SH2-like domain of Cbl. All Cbl coding sequences, except 358-906 c-Cbl are HAtagged. For targeting c-Cbl to the membrane, two forms of c-Cbl were generated. HA-c-CblCAAX was obtained by insertion of the farnesylation sequence of H-Ras, CMSCKCVLS, in frame in the C-terminus of c-Cbl after the stop codon. HA-N-Myr c-Cbl was made by inserting the c-Src myristoylation site, MGSSKSKPKDPSQRA, in the N-terminus of c-Cbl in frame before the start codon. Sub-cellular fractionation COS-7 cells were harvested 48 hours after transfection and fractionated as described (Hartley & Corvera, 1996). Briefly, the cells were resuspended in ice-cold hypotonic buffer and disrupted using a Dounce homogenizer. Tonicity was restored by the addition of hypertonic buffer. Unbroken cells were removed by centrifugation at 250g for 5 min at 4C, and nuclei were then precipitated at 750g for 5 min at 4C. The post-nuclear supernatant was centrifuged at 100,000g for 50 min at 4C in the presence of 5mM EDTA. The supernatant and the pellet were considered the cytosolic and the membrane fractions, respectively. The membrane pellet was solubilized using SDS sample buffer. For cell culture analyzed, the volume of the cytosolic fraction was 8.3 times higher than that of the membrane fraction. Equal volumes of each fraction were subjected to SDS-PAGE and immunoblotting. The amounts of proteins detected in the analyzed aliquots were quantified using the NIH Image software and multiplied by the corresponding fraction volume to determine their total amounts in each sub-cellular fraction. The purity of the fractions was controlled by immunoblotting for VLA-2a membrane markers, and either GAPDH or EF-1, cytosolic markers. Immunoprecipitation and immunoblotting Immunoprecipitation and immunoblotting was carried out as described previously (Feshchenko et al., 1998). Briefly, cells were lysed with ice-cold lysis buffer containing 1% NP40 and protease inhibitors. The lysates were clarified by centrifugation and incubated with the desired primary antibody. Immunoprecipitation of c-Cbl was carried out using Pansorbin (Calbiochem, La Jolla, CA), unless indicated otherwise. SLAP was immunoprecipitated with anti-FLAG antibody conjugated to agarose beads. The protein samples were resolved by SDSPAGE, and the blots were visualized using ECL Plus chemiluminescence kit (Amersham Pharmacia Biotech, Piscataway, NJ), and quantified using an Astra 1220S Scanner (UMAX, Fremont, CA) and the NIH Image or Un-scan-it (Silk Scientific, Orem, UT) software. For immunoprecipitation of c-Cbl from sub-cellular fractions, the membrane pellet obtained by ultracentrifugation, was solubilized using Octyl D-glucoside (ODG) lysis buffer: 50mM Tris, 150mM NaCl, 2mM EDTA, 2%ODG (pH 7.4) supplemented with the same inhibitors as the NP-40 buffer. An appropriate volume of concentrated ODG lysis buffer was added to the cytosolic fraction to have the same detergent concentration as the membrane fraction. Antibodies and Reagents The affinity-purified rabbit polyclonal antibodies to c-Cbl (C-15), CrkL (C-20), HA (Y11), goat polyclonal antibody to SLAP (C-19) and the anti-phosphotyrosine mouse monoclonal antibody (PY-20), were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The rabbit polyclonal antiserum to p85-PI3-Kinase and the mouse monoclonal antibody to EF-1 Protein G-agarose beads were purchased from UBI (Lake Placid, NY). Anti-GAPDH monoclonal antibody (6C5) was purchased from Research Diagnostic (Flanders, NJ). Anti-VLA-2 - Cbl monoclonal antibodies were purchased from BD Biosciences (San Diego, CA). Anti-FLAG M2, unconjugated and agarose-conjugated monoclonal antibody and anti-FLAG rabbit polyclonal antibody were purchased from SIGMA. Chicken SLAIgY antibody was obtained form Genway Biotech (San Diego, CA). (Goat and chicken anti-SLAP antibodies were used to detect SLAP in lysates of COS-7 and v-Abl/3T3 cells, respectively). HRP-conjugated secondary antibodies were purchased from Amersham or Santa Cruz Biotechnology. Human plasma fibronectin was purchased from Invitrogen. Cell Spreading assay Cells were plated at 20-30% confluency, in duplicate, on FN-coated plates for the required time. Phase-contrast images of live cells were taken under 20X objective using NIKON DXM 1200 microscope and NIKON ACT-1 software. The area covered by the cells was measured using Photoshop image software. At least 80 cells were analyzed per cell type. A cell was considered spread when its area was at least 2-fold greater than the average area of a round cell. Quantitation results were presented as percentage of spread cells. Cell Adhesion assay Cells were plated in duplicate in 12-well tissue culture plates for the required time. The culture supernatant containing non-adherent cells was removed, followed by two gentle washes with 1 ml of growth medium to remove loosely attached cells. Adherent cells were recovered by treatment with cell stripper (Mediatech, Herndon, VA) for 10-15 minutes, followed by two washes with 1 ml of growth medium to ensure complete recovery of the adherent population. The cell suspensions were centrifuged at 350g for 5 minutes at 25C. Adherent and non-adherent cells were resuspended in appropriate volume of growth medium and counted using a hemocytometer. The percentage of adherent cells was calculated as follows: Adherent Cells (%) = 100% x Adherent cells / (Non-adherent+Adherent) cells. Cell Migration Cell migration assays were carried out using modified Boyden chamber as described (Teckchandani et al., 2005b). Briefly, the assay was performed in a 48-well chemotaxis chamber (Neuroprobe, Gaithersburg, MD) with PVP-free polycarbonate filters (pore size-8µm, VWR, West Chester, PA) coated with 10g/ml of human plasma FN, sandwiched between the upper and lower chambers. v-Abl-3T3 cells expressing c-Cbl alone or c-Cbl with SLAP or its mutants were resuspended in DMEM containing 0.2% fatty acid-free BSA and added to the wells in the upper chamber. Serum diluted in DMEM (10%), a chemotactic agent, was added to the wells in the lower chamber. The cells were allowed to migrate for about 10 hours at 37C. The filter was removed and the unmigrated cells on the topside were scraped. The migrated cells on the underside of the filter were fixed in methanol and stained with eosin/thiazine. Each condition was analyzed in duplicate and three individual fields were counted under 20X magnification using Image Pro software (Media Cybernetics, Silver Springs, MD). Statistical analysis of data Statistical analysis of data was carried out using the GraphPad Prism software (GraphPad, San Diego, CA).