Read full Article
advertisement

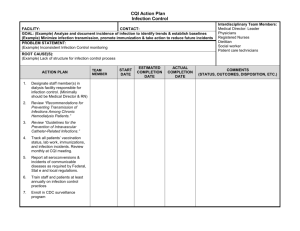
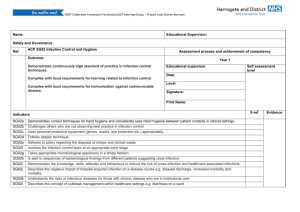
ISRAEL JOURNAL OF VETERINARY MEDICINE VOLUME 54 (3) 1999 DETECTION OF IL-12 ON PERIPHERAL BLOOD MONONUCLEAR CELLS OF BOVINE LEUKEMIA VIRUSINFECTED CALVES BY FACS ANALYSIS H. Ungar-Waron, R. Paz, J. Brenner and Z. Trainin Department of Immunology, Kimron Veterinary Institute, 50250 Beit Dagan, Israel Abstract An experimental model of bovine leukemia virus (BLV) infection was established in young calves. All animals used in the experiment became infected but the patterns of cellular response varied among individual animals during infection. At early stages of infection a short-termed IL-12p40 gene expression in peripheral blood mononuclear cells (PBMC) was observed which seemingly directed the subsequent stages of infection. We were able to demonstrate that in addition to IL-12p40 cytokine gene expression, the presence of the cytokine on PBMC of infected calves could be detected by means of flow cytometry studies (FACS analysis). The transient nature of its appearance made the kinetics of its manifestation difficult to follow and not in all the calves was it possible to assess the participation of this key cytokine of cellmediated immunity in the immunopathology of BLV retroviral infection. Introduction In a previous paper, we reported on short-termed expression of interleukin12 (IL-12) during experimental BLV infection (1). This cytokine plays a central role in the regulation of Th1 and Th2 cellular responses. Whereas Th1 cells are involved in cellular immunity, Th2 cells address functions of the humoral response. Optimal generation of Th1 CD4+ T cells and CD8+ cytotoxic T lymphocytes (CTL) requires IL-12 expression (2). Because of the critical role of IL-12 during different infections, the immune response in Th1-mediated pathogen resistance can be distinguished by the kinetics of IL-12 production (3). We have established an experimental model of BLV infection (4). This retrovirus induces a chronic infection in cattle leading in some cases to persistent lymphocytosis (PL+) due to intensive polyclonal B-cell expansion (5). In this model the early stages of infection could now be investigated and, in particular, levels of cytokine expression. However, no uniform pattern of cell proliferation nor of cellular immune response was observed in infected calves throughout experimental infection (1,4). A short-termed IL-12p40 gene expression was observed in PBMC of two out of 4 infected animals, one to 3 weeks after infection. It was of interest to examine whether expression of the IL-12p40 protein molecule on the cell surface of BLV-infected PBMC could possibly be detected while it was being secreted. Materials and Methods Experimental animals Six Friesian-Holstein, 7 months old, steers were employed. They were clinically healthy and BLV-free at the beginning of the experiment. Four calves (numbers, 056, 077, 714, 715) were infected by intravenous injection (jugular) with 106 bovine embryo kidney cells (BEK) persistently infected with BLV (BEK-BLV). This cell line was obtained as described (1). Two other calves were mock-infected each with 106 BEK cells co-cultivated with normal PBMC. Monoclonal antibody to IL-p40 This is a product of PharMingen (20711D) developed for specifically measuring human IL-12p40 subunit in an ELISA capture assay and was used in this study for flow cytometry. Enumeration of blood lymphocytes Peripheral blood was collected by venipuncture into tubes containing EDTA as anticoagulant, and total leukocyte counts were determined on an automated cell counter (Technicon H. 1ETM System, Miles Tarrytown, NY) using the manufacturer’s instructions. Detection of antibodies to BLV A standard diagnostic test, AGID, as recommended by the Office International des Epizooties, (1992), was used (6). Serum samples were tested for precipitating antibodies to commercially available BLV glycoproteins (Pittman-Moore, Atlanta, GA) which contained both the Mr 51000 BLV envelope glycoprotein (BLV-gp51) and a small amount of Mr 24000 viral core protein. Flow cytometry (FACS analysis) PBMC were obtained from each blood sample by Ficoll-Hypaque centrifugation, and 106 cells were incubated with the anti IL-12p40 specific mAb for 30 min at 40C. After washing, FITCconjugated (Fab’)2 anti-mouse IgG (Fc portion) was added at an appropriate dilution and incubated as before. After additional washings, cytofluorographic analysis was performed in a fluorescence-activated cell analyzer (FACScan, Becton-Dickinson, CA). Results and Discussion Establishment of an experimental model of BLV infection in calves Four calves were experimentally infected with BLV as described. Infection with BLV was assessed by detection of antibodies to BLVgp51 antigen in the sera of all the experimentally infected animals by AGID from the fourth to fifth week after infection. These persisted to the end of the experiment. Infection was likewise assessed either by BLVgp51 mRNA expression or by DNA amplification to detect provirus-infected cells as reported elsewhere (1). Mockinfected calves remained BLV-PL- negative to the end of the experiment, 6 to 12 months later. In two calves (056 and 715), lymphocytosis developed gradually (Table 1) and PL+ positive was established permanently after 4 weeks. Transient lymphocytosis between the third and fifth week after infection was observed in calf 714. PBMC counts stayed low and the state of PL- negative persisted for at least 3 months but it eventually became PL+ by the end of the experiment. Calf 077 stayed PL negative for 11 weeks and became PL+ after 4.5 months. IL-12p40 We have previously reported (1) that two BLV experimentally infected calves, 056 and 077, exhibited strong IL-12p40 mRNA expression one to three weeks after infection which decreased to undetectable levels by 12 weeks and was not detected further before the end of the experiment 18 months later. However when PBMC of these animals were stimulated with conA, IL-12p40 gene expression was detected throughout the entire experimental period. Six months after infection, PBMC of calves 056 and 077, were labeled with anti IL-p40 monoclonal antibody and examined by FACS analysis. As seen in Figure 1, 17 to 19 percent of PBMC were IL-12p40 positive. In PBMC of the two other calves, 714 and 715, IL-12p40 gene expression was not detected at any stage of the infection. However by FACS analysis it was possible to detect a noticeable amount of the IL-12p40 on PBMC of calf 714 two weeks after infection which gradually decreased during the following weeks (Table 2). No such reactivity was detected in PBMC of calf 715. We conclude that the kinetics of expression of IL-12p40 cytokine gene in PBMC or its detection on their surface have no direct connection with lymphocyte counts or the state of infection of the infected calves. IL12-p40 mRNA expression and protein secretion have been demonstrated in human monocytes that were activated by Brucella abortus (7). Similarly, IFN-gamma protein was detected in supernatants of rat peritoneal mast cells following IL-12 treatment (8). Thus both cytokine gene expression and protein secretion can be studied in various systems. Detection of IL-12p40 on PBMC of experimentally BLV-infected calves is an example of such a system in which its transient presence is identified possibly as a step in its secretion. Acknowledgement This project was supported by US-Israeli Binational Agriculture Research and Development Fund. (BARD) US-2367-94. References 1. Yakobson, B., Brenner, J., Ungar-Waron, H. and Trainin, Z. 1998. Shorttermed expression of interleukin-12 during experimental BLV infection may direct disease progression to persistent lymphocytosis. Vet. Immunol. Immunopathol. 64: 207-218. 2. Trinchieri, G. 1995. Interleukin-12: A pro-inflammatory cytokine with immunoregulatory function that bridge innate resistance and antigen specific adaptive immunity. Ann. Rev. Immunol. 13: 251-276. 3. Gorham, J., Guteer, M. and Murphy, K. 1997. Genetic control of interleukin 12 responsiveness: implication for disease pathogenesis. J. Mol. Med. 75: 502-511. 4. Ungar-Waron, H., Paz, R., Brenner, J. Yakobson, B., Partosh, N and Trainin, Z. 1999. Experimental infection of calves with bovine leukemia virus (BLV): an applicable model of a retroviral infection. Vet. Immunol. Immunopathol. 67: 195-201. 5. Schwartz, I. and Levy, D. 1994. Pathology of bovine leukemia virus. Vet. Res. 25: 521-536. 6. Office International des Epizooties (OIE), 1992. Enzootic bovine leukosis. In Manual of Standards for Diagnostic Tests and Vaccines. OIE, Paris, 305312. 7. Zaitseva, M., Golding, H., Manischewitz, J., Webb, D., and Golding, B. 1996. Brucella abortus as a potential vaccine candidate: induction of IL-12 secretion and enhanced B7.1 and B7.2 and intercellular adhesion molecule 1 surface expression in elutriated human monocytes stimulated by heatinactivated B.abortus. Infection and Immunity. 64: 3109-3117. 8. Gupta, A., Leal-Berumen, I., Croitoru, K. and Marshall, J.S.1996. Rat peritoneal mast cells produce IFN-gamma following IL-12 treatment but not in response to IgE-mediated activation. J. Immunol. 157; 3123-2128