Thermochemistry, Thomas - HighSchoolScienceTFALA
advertisement

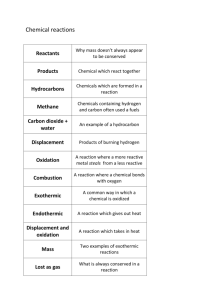
UCLA, GK-12 Science & Mathematics in Los Angeles Urban Schools http://www.nslc.ucla.edu/STEP/GK12/ Thermochemistry Developed by: K. Thomas, A. Knudson & J. Ta. Teacher Material California State Standards Chemistry, 7b. Students know chemical processes can either release (exothermic) or absorb (endothermic) thermal energy. Investigation and Experimentation, 1a. Select and use appropriate tools and technology to perform tests, collect data, analyze relationships, and display data. Investigation and Experimentation, 1c. Identify possible reasons for inconsistent results, such as sources of error or uncontrolled conditions. Investigation and Experimentation, 1d. Formulate explanations by using logic and evidence. Synopsis The purpose of this inquiry-based lab/lesson is to have students discover endothermic and exothermic reactions by determining if calcium chloride or ammonium nitrate would be good ingredients for hot packs or cold packs. Background From this lab, students should develop an understanding of exothermic and endothermic reactions and know that chemical reactions can be classified as endothermic or exothermic. Exothermic reactions are reactions that transfer energy to the surroundings. The energy is usually transferred as heat energy, causing the reaction mixture and its surroundings to get hotter. So, a reaction in which heat is given off is called exothermic.. In an exothermic reaction the total energy absorbed in bond breaking is less than the total energy released in bond making. Exothermic reactions can be written as: Reactants → Products + Energy. Exothermic processes release heat and will feel hot. In this lesson, we will mix calcium chloride with water to observe an exothermic reaction. Endothermic reactions are reactions that take in energy from the surroundings. The energy is usually transferred as heat energy, causing the reaction mixture and its surroundings to get colder. So, if heat is absorbed in a reaction, the reaction is said to be endothermic.. An endothermic reaction is one that requires more heat to break the bonds of the reactants than is gained in new bonds of the products.In other words, the reaction absorbs heat, causing its surroundings to get colder. Endothermic reactions can be written as: Reactants + Energy → Products. Endothermic processes absorb heat and will feel cold. In this lesson, we will mix ammonium nitrate with water to observe an endothermic reaction. Page 1 of 7 UCLA, GK-12 Science & Mathematics in Los Angeles Urban Schools http://www.nslc.ucla.edu/STEP/GK12/ Objectives 1. Students will be introduced to hot and cold packs. 2. Students will carry out two reactions, one that is endothermic and one that is exothermic. 3. Students will become more familiar with the scientific method; students will make observations and hypotheses, follow a procedure, collect and record data, graph data, and make conclusions. 4. Students will develop writing skills by completing the required sections for each experiment. 5. Students will develop graphing skills by graphing their data and interpreting the graph. Suggested Timeline Day one. Introduction and student experiments. 50-60 minutes. Day two. As this lesson is designed only to introduce the students to endothermic and exothermic reactions, it should be followed by a detailed discussion of endothermic and exothermic reactions that covers the details of the standard. Materials (for each group) Ammonium nitrate (NH4NO3) Aprons (optional) Calcium chloride (CaCl2) Gloves (optional) Goggles Paper cups (for chemicals) Paper towels Stirring stick Stopwatch Styrofoam cups Thermometer Water You will also need a beaker (or two) that the students can use to measure 100mL of water and a balance that you can use to weigh the chemicals (see below). Have enough hot and cold packs on hand to show every class. Hot packs will last several hours while cold packs will last only an hour or so. Most of the cold packs you buy in stores consist of ammonium nitrate and water. Hot packs, however, are usually air activated rather than a mixture of CaCl2 and water. Nonetheless, they are an example of an exothermic reaction. For each reaction, the students will use 100mL of water. For the chemicals, they will need approximately 40g of calcium chloride and 30g of ammonium nitrate (a slightly higher or lower concentration will still produce the desired effect). While we prepared the chemicals for them, you could have the students weigh the chemicals themselves. Teaching Tips Outline for activity: Hold up and introduce hot and cold packs. Ask the class: o What are they used for? o What do they do? o How do they work? Start the reactions and pass the packs around the room- tell students they get three seconds to hold a pack before they pass it along. Page 2 of 7 UCLA, GK-12 Science & Mathematics in Los Angeles Urban Schools http://www.nslc.ucla.edu/STEP/GK12/ Tell the class: in today’s activity, we are going to discover if two chemicals would be good ingredients in a hot or cold pack. Ask the following questions and write their responses to the last three on the board under the titles “materials”, “safety”, and “data”. o How can we find this out? (mix with water) o How will you know if a chemical is a good chemical to use in a hot pack?...in a cold pack? (gets hot or cold) o What materials do you need? (water, thermometer, container, …) o What about materials for safety? (goggles, gloves, aprons) o What kind of data should you record? (temperature, time) Tell the class: first, let’s make some predictions. Here are the two chemicals we have to work with and some of their characteristics (show power point slide): Pass out the handouts. Have the students use the information presented on the slide as their observations. Tell them to write down what they think are important observations about these chemicals- observations that will help them make predictions that will answer the questions. Tell the class: based on what you know about these chemicals, make some predictions (have them write their hypotheses): o What do you think will happen when you mix Calcium Chloride with water? o What do you think will happen when you mix Ammonium Nitrate with water? o Will either of these chemicals be good candidates for an ingredient in a hot pack? In a cold pack? Why? Once they have completed the observations and hypotheses sections, they can start the experiments. Review with them the protocol and handout the role cards. You may need to review Celsius vs. Fahrenheit and show them how to read the thermometers. They should completely finish one reaction and clean up before they start the next. Roles Page 3 of 7 UCLA, GK-12 Science & Mathematics in Los Angeles Urban Schools http://www.nslc.ucla.edu/STEP/GK12/ We created role cards for each group so that each student had responsibilities and so they could refer to the cards during class. The roles were as follows: 1. Get water and record data: Your job is to get 100ml of water in your Styrofoam cup and bring it back to the group. Then, when your group starts recording the temperature, you will write the data down. The last reading will be at 3:00. 2. Get chemicals and timer: Your job is to get the chemicals (one at a time) from your teacher (in small paper cup) and take them back to your group. As soon as the chemical is added to the water, start the stop watch and say “read” every 15 seconds. This will let your group know that it is time to read the temperature. When the stop watch reaches 3:00, say “read” and then tell your group that you have reached three minutes. 3. Mixer and cup holder: After your group has measured the temperature and everyone is ready, you will add the chemical to the water and stir continuously with the stir stick. It is also your job to hold the Styrofoam cup to make sure it doesn’t tip over (very important!). 4. Thermometer reader and holder: It is your job to read the initial temperature of the water (in Celsius) and then to read the temperature every 15 seconds when directed by your team. As you do this, make sure you hold the thermometer in the liquid. The last reading will be at 3:00. Clean up: After each experiment, they should dump the liquids into separate, marked basins (alternatively, the solutions can be flushed down a sink with plenty of water), throw the cups in the trash, and wipe the thermometer off with the paper towel. Homework (or in class, if time): 1. Graph how the temperature changed for each chemical. On your graph, the xaxis will be time and the y-axis will be temperature. Make sure you label your axes and give your graph a title. 2. Answer the conclusion questions on your handout. Page 4 of 7 UCLA, GK-12 Science & Mathematics in Los Angeles Urban Schools http://www.nslc.ucla.edu/STEP/GK12/ (STUDENT HANDOUT BEGINS) THERMOchemistry STEP 1: QUESTIONS 1. Is calcium chloride (CaCl2) a good candidate (choice) for an ingredient in a cold pack? Is it a good candidate for an ingredient in a hot pack? 2. Is ammonium nitrate (NH4NO3) a good candidate (choice) for an ingredient in a cold pack? Is it a good candidate for an ingredient in a hot pack? STEP 2: OBSERVATIONS 1. Calcium chloride (CaCl2): a.________________________________________________________________ b.________________________________________________________________ c.________________________________________________________________ 2. Ammonium nitrate (NH4NO3): a.________________________________________________________________ b.________________________________________________________________ c.________________________________________________________________ STEP 3: HYPOTHESES 1. What do you think will happen when you mix calcium chloride (CaCl2) with water? Why?______________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ 2. What do you think will happen when you mix ammonium nitrate (NH4NO3) with water? Why?________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ 3. Will either of these chemicals be good candidates for an ingredient in a hot pack? Why or why not? _____________________________________________________ ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ 4. Will either of these chemicals be good candidates for an ingredient in a cold pack? Why or why not? _____________________________________________________ ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ Page 5 of 7 UCLA, GK-12 Science & Mathematics in Los Angeles Urban Schools http://www.nslc.ucla.edu/STEP/GK12/ STEP 4: MATERIALS AND PROCEDURE MATERIALS: Ammonium nitrate (NH4NO3) Goggles Aprons (optional) Paper cups (for chemicals) Calcium chloride (CaCl2) Paper towels Gloves (optional) Stirring stick Stopwatch Styrofoam cups Thermometer Water PROCEDURE: 1. Using a beaker, measure 100 mL of water into a Styrofoam cup. 2. Measure the temperature of the water and record it in your data table. 3. Using a small paper cup, obtain approximately 40 g of calcium chloride (CaCl2) from your teacher. 4. Add the chemical to the water and start the stop watch immediately. Be sure to stir. 5. Record the temperature every 15 seconds until you reach 3 minutes. 6. Dispose the solution in the correct labeled bin and throw the cups away. 7. Wipe the thermometer off with a paper towel. 8. Repeat steps 1-7 using 30 g of ammonium nitrate (NH4NO3). STEP 5: DATA COLLECTION 1. For each reaction, record the temperature of the water every 15 seconds in the table below. 2. Graph your data. Label the horizontal x-axis “time” and the vertical y-axis “temperature”. You will plot one line for calcium chloride (CaCl2) and one line for ammonium nitrate (NH4NO3). Be sure to title your graph and make a key. calcium chloride (CaCl2) Time (min:sec) Temperature (˚C) 0 ammonium nitrate (NH4NO3) Time (min:sec) Temperature (˚C) 0 0:15 0:15 0:30 0:30 0:45 0:45 1:00 1:00 1:15 1:15 1:30 1:30 1:45 1:45 2:00 2:00 2:15 2:15 2:30 2:30 2:45 2:45 3:00 3:00 Page 6 of 7 UCLA, GK-12 Science & Mathematics in Los Angeles Urban Schools http://www.nslc.ucla.edu/STEP/GK12/ STEP 6: CONCLUSIONS 1. Using your graph, summarize what happened when you added calcium chloride (CaCl2) to water. ___________________________________________________ _________________________________________________________________ _________________________________________________________________ _________________________________________________________________ 2. Using your graph, summarize what happened when you added ammonium nitrate (NH4NO3) to water. _________________________________________________ _________________________________________________________________ _________________________________________________________________ _________________________________________________________________ 3. a. Which of the chemicals would be a good candidate for a hot pack? Why? ____ _________________________________________________________________ _________________________________________________________________ _________________________________________________________________ b. Does this match your hypothesis? _________ 4. a. Which of the chemicals would be a good candidate for a cold pack? Why? ____ _________________________________________________________________ _________________________________________________________________ _________________________________________________________________ b. Does this match your hypothesis? _________ 5. Chemicals can store and release energy in the form of heat. A chemical reaction that releases heat (and becomes hot) is called an exothermic reaction. But chemical reactions can also absorb heat from the environment and become cold. These reactions are called endothermic reactions. When chemicals are dissolved in water, sometimes heat is released and sometimes heat is absorbed. a. How do you know if a reaction is exothermic? ________________________ _____________________________________________________________ _____________________________________________________________ b. How do you know if a reaction is endothermic? _______________________ _____________________________________________________________ _____________________________________________________________ 6. Which of these reactions is exothermic? ___________________________________ 7. Which of these reactions is endothermic? _________________________________ (STUDENT HANDOUT ENDS) Page 7 of 7