Lecture 8
advertisement
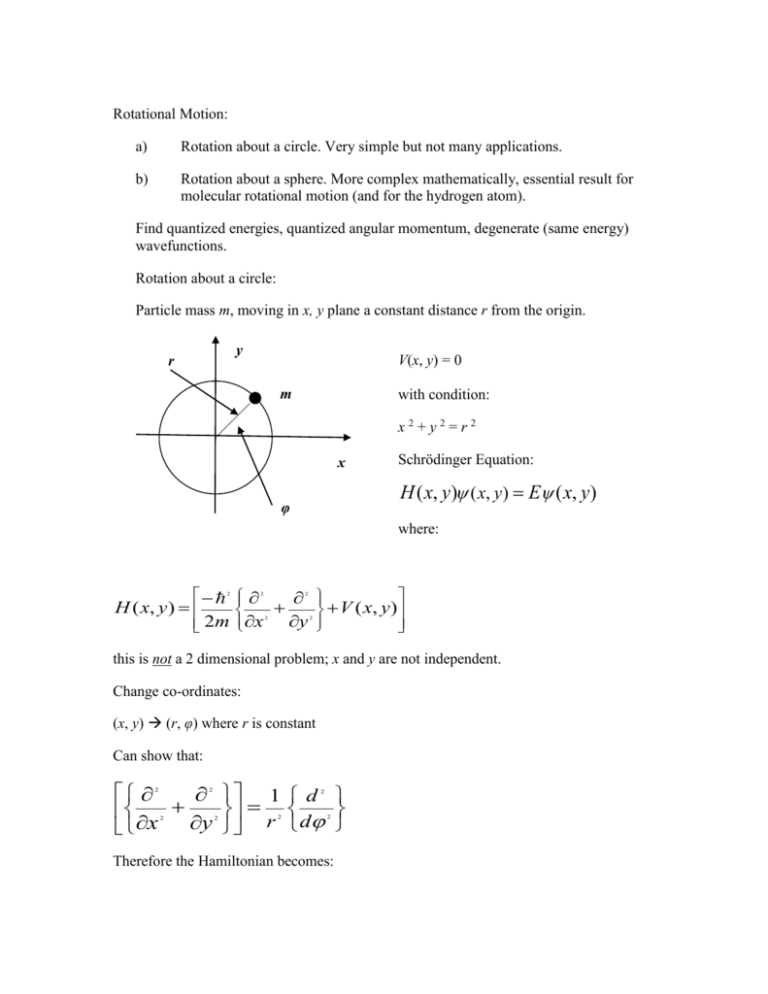
Rotational Motion: a) Rotation about a circle. Very simple but not many applications. b) Rotation about a sphere. More complex mathematically, essential result for molecular rotational motion (and for the hydrogen atom). Find quantized energies, quantized angular momentum, degenerate (same energy) wavefunctions. Rotation about a circle: Particle mass m, moving in x, y plane a constant distance r from the origin. y r V(x, y) = 0 m with condition: x2+y2=r2 x Schrödinger Equation: H ( x, y) ( x, y) E ( x, y) φ where: H ( x, y ) 2m 2 x 2 y 2 2 2 V ( x, y ) this is not a 2 dimensional problem; x and y are not independent. Change co-ordinates: (x, y) (r, φ) where r is constant Can show that: 1 d r d x y 2 2 2 2 2 2 2 Therefore the Hamiltonian becomes: d 2mr d H ( ) and 2 2 2 2 V ( ) V ( ) 0 for all in the Schrödinger Equation H ( ) ( ) E ( ) d 2mr d 2 2 2 E 2 d 2mr d 2 2 2 E 2 A exp ik , k 2mr 2 E 2 1/ 2 Boundary Condition: The wavefunction must obey ( ) (2 ) otherwise it will interfere destructively with itself! 2 A exp ik A exp ik 2 A exp ik exp ik 2 1 exp ik 2 k 0,1,2,3... Again, quantization arises from the Boundary Conditions. 2mr E k k E , k 2mr 2 1/ 2 2 2 k 2 2 the wavefunctions are: 0,1,2,3... A exp ik , k integer 2 2 d 2 0 * d 2 A e e d ik 2 0 ik 0 A d A 2 1 2 2 2 0 A 2 2 1 / 2 1 / 2 k exp ik , k integer Notes: 2 , E 0 the particle is equally probable at all values of . 1) k = 0 is allowed, 2) 1 / 2 0 0 and have the same energy E k k . They are degenerate. k l E 2mr 2I 2 3) What is different? k k 2 2 2 l – Angular momentum, I – moment of inertia l l , l k and is different (and quantized). 2 2 l = +k(h/2π) Different rotation directions. l = -k(h/2π) How does this connect to (say) a diatomic molecule with fixed bond length, rotating in the x, y plane? Rotation on a sphere. Particle mass m V(x, y, z) = 0 if x2 + y2 + z2 = r2 2 dimensional problem. Change coordinates (x, y, z) (θ, φ) H , y z m r m x is called the Legrengian and the solution of the 2 2 2 2 2 2 2 , 2 2 2 2 H ( , ) , ( , ) E ( , ) m r 2 2 2 is mathematically fairly complex. Results: E 2 l (l 1), l 0,1,2 2mr ( , ) f ( ) exp( im ), l m l l l ,m 2 l ,m Each energy level is (2l + 1) degenerate. 2 quantum numbers l and m. l defines the angular momentum of the particle, m defines the direction of the angular momentum. l E l (l 1) 2mr 2mr angular momentum, l l (l 1) l 0,1,2 2 2 2 l 2 2 1/ 2 Angular momentum is quantized. Relationship to a diatomic molecule, fixed bond length R, moving in 3 dimensional space. 2 particles, m1 at (x1, y1, z1) and m2 at (x2, y2, z2), (x1 – x2)2 + (y1 – y2)2 + (z1 – z2)2 = R2 V(x1, y1, z1, x2, y2, z2) = 0 Change coordinates (x1, y1, z1, x2, y2, z2) (x, y, z, θ, φ) (x, y, z) – centre of mass m y z m x y z x , x y z R m m total mass reduced mass translation rotation 2 2 2 2 1 2 1 2 2 2 2 1 2 2 2 1 2 2 2 2 2 2 2 2 2 2 2 2 Can separate translation from rotation. 2 2 2 2